Absence of Cortical Gray Matter Abnormalities in Psychosis of Epilepsy
Abstract
The authors retrospectively explored cortical differences between 26 patients with temporal lobe epilepsy and psychosis of epilepsy (POE), 24 patients with temporal lobe epilepsy (TLE) alone, and 20 healthy comparison subjects. Using voxel-based morphometry based on statistical parametric mapping (SPM99), which is an unbiased and fully automated technique to test for morphometric differences, magnetic resonance imaging (MRI) 3D-datasets were acquired and analyzed. There were no significant cortical gray matter differences between the POE and the TLE group. Since cortical pathology is prominent in schizophrenia, POE may be a clinical entity separate from schizophrenia.
Psychosis is a severe complication of epilepsy with a prevalence between 0.5% and 9%.1–3 The symptomatology is often reminiscent of schizophrenia, but clinically POE is distinguished by the lack of negative symptoms and better premorbid and long-term functioning.4
In terms of etiology, little is known about the pathogenesis of POE. Since POE classically follows the onset of epilepsy with a latency of several years and it is more common in temporal lobe epilepsy (TLE), Slater and colleagues put forward the temporal lobe hypothesis of POE.5 Since then, POE has been related to early onset of epilepsy,6 bitemporal seizure foci,7 temporal lobe dysplasias,8 and foreign body lesions.9
Recently, we reported amygdala enlargement in a volumetric study of 26 patients with POE, distinguishing this patient group from patients with TLE alone and healthy controls.10 With volume loss of mesial temporal lobe structures (hippocampus and amygdala) being one of the most consistent findings in schizophrenia,11 this observation supported the notion that schizophrenia, similar to psychosis of epilepsy, is a distinct nosologic entity. However, there is no agreement as to whether the pathophysiology of POE is similar to that of schizophrenia. Furthermore, POE might as well result from a comorbidity between schizophrenia and TLE.10,12
In schizophrenia, apart from volume loss of subcortical structures such as the hippocampus and amygdala, there are many reports of cortical abnormalities. A majority of magnetic resonance imaging (MRI) studies in schizophrenia found cortical abnormalities, particularly in the temporal,13 prefrontal,14 and also parietal15 cortex. Recently, Wilke et al.16 reported left dominant frontal-temporal and insular gray matter reduction in schizophrenia, using statistical parametric mapping to analyze the differences. Using the same method, Ananth and et al.17and Hulshoff Pol et al.18 found decreases of gray matter density in several cortical areas, including the frontal and temporal cortex.
In this study, we wanted to test the hypothesis that, similar to schizophrenia, there is evidence of cortical gray matter reduction in patients with TLE and POE. In doing so, we studied the same patient sample as the one published recently.10 However, this time we used the methodology of voxel-based morphometry (VBM) in the framework of statistical parametric mapping to analyze possible cortical abnormalities in this condition. This methodology is completely different to the one used in the previous study, where manual tracing of predefined subcortical structures like the amygdala and hippocampus was used to examine possible differences between POE and schizophrenia. In contrast, this study reports results of VBM, which is an unbiased and fully automated approach to analyze differences in cortical gray matter density.
Rationale of the Study
In summary, we employed the objective and fully automated technique of VBM to test the hypothesis that, similar to schizophrenia, there is evidence of widespread frontotemporal cortical gray matter abnormalities in POE. More precisely, we wanted to answer the following questions:
Do patients with POE differ from patients with TLE alone and healthy comparison subjects in terms of frontotemporal gray matter density?
Is there any significant difference between patients with schizophrenia-like psychosis of epilepsy (SLPE) and postictal psychosis (PIP) and control groups?
Is there any significant interaction between variables like age, intelligence quotient (IQ), hippocampal and amygdala volumes, and cortical gray matter density in the different study groups?
METHODS
Patient Identification
Approval for this study was obtained from the ethics committee of the National Hospital for Neurology and Neurosurgery (NHNN), University College London Hospitals, Queen Square, London, United Kingdom (UK). Our study used a retrospective approach to identify patients with temporal lobe epilepsy at a tertiary referral centre (National Society for Epilepsy, Chalfont Centre for Epilepsy, UK). The clinical syndrome of interest was defined as complex partial seizures with clinical features and electroencephalogram (EEG) and MRI findings compatible with temporal lobe epilepsy. All patients with chronic intractable TLE who had been referred to the Chalfont Epilepsy Centre from 1995-1999 (n = 1,008) were surveyed, and a total number of 26 patients (2.6%) with POE were included. Full hospital notes were obtained for these cases, and patients were then classified into groups of postictal psychosis and interictal psychosis on the basis of notes made from discharge summaries from inpatient stays at psychiatric hospitals and/or by consultant psychiatrists working in the Department of Neuropsychiatry, National Hospital. The minimum requirement for a diagnosis of psychosis was the presence of delusions and/or hallucinations. Confusional states or depressive symptoms alone were not regarded as sufficient. The temporal relationship of the psychotic mental state to the ictus was noted, and patients that presented with psychopathology independently of seizures were classified as suffering from an interictal schizophrenia-like psychosis (SLPE), while psychotic episodes that appeared in a clear temporal relationship to a cluster of seizures, mostly being separated from it by a lucid interval, were said to represent postictal psychoses (PIP). Episodes that seemed to be drug-induced, provoked by excessive consumption of alcohol, or represent complex partial status were excluded. These criteria closely follow the description of postictal psychosis by Logsdail and Toone.19 TLE diagnoses were made by neurologists not involved in this study. Patients with extratemporal or generalized epilepsy were excluded, as were patients with a full IQ below 70 on the Wechsler Adult Intelligence Scale-Revised (for patient selection and classification of postictal and interictal psychosis, see Tebartz van Elst et al.10 and Figure 1; for demographic and clinical patient characteristics see Table 1.)
The two control groups consisted of 24 randomly chosen cases of temporal lobe epilepsy without any psychopathology except dysthymia, who were group-matched for age, sex, duration of epilepsy and antiepileptic medication, and 20 healthy volunteers.
Image Acquisition
Magnetic resonance imaging was obtained at the Chalfont Centre for Epilepsy on a 1.5T GE Signa scanner (GE Medical Systems, Milwaukee, Wisc.) using a T1-weighted inversion-recovery prepared volume acquisition [IRSPGR: TI/TR/TE/flip = 450/15/4.2/20; 124 × 1.5 mm thick contiguous coronal slices; matrix 256 × 192, 24 cm × 18 cm (field of view; TI = inversion time; TR = repetition time; TE = echo time)]. Conventional spin echo sequences [TR/TE1/TE2 2000/30/120/, number of excitations = 1, 256 × 192 matrix, 24 × 18 cm for 5 mm thick coronal slices with no gap; scan time 10 min (NEX = number of excitations)] were obtained for the radiological diagnoses, which were made by two neuroradiologists following visual assessment of the MRI scans.
Preprocessing of Structural Data
Template creation.
An anatomical template was created from 31 healthy subjects imaged on the same MRI scanner, with the same scanning parameters in order to reduce scanner-specific bias. This process involved spatially normalizing each structural MRI to the ICBM 152 template (Montreal Neurological Institute) which is derived from 152 normal subjects and approximates the Talairach space. The normalized data were then smoothed with a 6-mm-full-width at one-half-maximum (FWHM) isotropic Gaussian kernel, and the template was created as a mean image.
Spatial normalization.
All scans in native space were transformed to the same stereotactic space. The first step in spatial normalization consists of estimating the optimal 12-parameter affine transformation to match images.20 In a Bayesian framework, the maximum a posteriori estimate of the spatial transformation is made using prior knowledge of normal variability in brain size. The second step accounts for global nonlinear shape differences that are modeled by a linear combination of smooth basal functions.21 This normalization allows the detection of local differences in the concentration of gray matter, having discounted global shape differences.22 The spatially normalized images were resliced with a final voxel size of 1 × 1 × 1 mm3 (recommended by J. Ashburner, oral communication).
Segmentation.
Scans were then segmented into gray matter, white matter, cerebrospinal fluid (CSF), and other nonbrain partitions. Statistical parametric mapping (SPM) segmentation uses a mixture model cluster analysis to identify voxel intensities matching particular tissue types (gray matter, white matter, and CSF) combined with an a priori knowledge of the spatial distribution of these tissues in normal subjects, derived from probability maps.20
Brain extraction.
This is a fully automated procedure in SPM99 to remove scalp tissue, skull, and dural venous sinus voxels. In a recent large study, Good et al.23 showed that without a brain extraction step, there appear to be significant differences in gray matter that are in fact due to misclassified nonbrain tissue.
Smoothing.
The normalized, segmented, and extracted images were smoothed with a 12-mm FWHM isotropic Gaussian kernel, which renders the data more normally distributed, and thus increases the validity of the parametric statistical tests in SPM.
Statistical Analysis
We analyzed the normalized, segmented, extracted and smoothed images by statistical parametric mapping (SPM99) in the framework of the General Linear Model.24 Hypotheses about regionally specific effects between groups by two contrasts were tested. This analysis detects whether each voxel has a greater or lower gray matter density in a patient group than in the other patient group or the healthy comparison group. To control for differences in voxel intensity across scans, global mean voxel value was included in all analyses as a confounding covariate in an analysis of covariance (ANCOVA), while preserving regional differences in gray and white matter.24
We compared 1) the group of TLE and POE versus the group of TLE alone in order to test our main hypothesis; 2) the whole group of TLE alone versus healthy subjects in order to check the sensitivity of our method; 3) subsamples of the three main groups, which were homogeneous with respect to EEG laterality and magnetic resonance (MR) pathology, versus each other in order to control for possible differences between subgroups that were specific to EEG or MR findings; and 4) the PIP group with the SLPE group. In a final set of analyses, age, IQ, and amygdala, hippocampal, and total brain volumes, measured in a previous study of the same sample,10 were included as additional covariates in order to account for possible confounding effects of these variables.
Significance levels were set at p < 0.05, with p being corrected for voxel-by-voxel comparisons in the entire volume. Corrections for the search volume and implicit multiple voxel-wise comparisons were calculated using the Gaussian random field theory, which is the established approach to inference in smooth spatially extended data.25
RESULTS
EEG and MRI Abnormalities
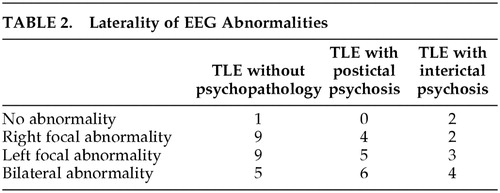
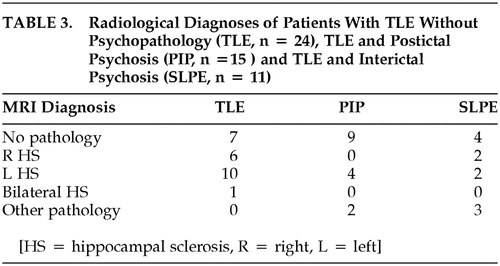
There was no difference in the distribution of EEG abnormalities between the groups, with respect to laterality. While hippocampal sclerosis was more common in patients without POE, there were no significant differences in terms of nature and laterality of the neuropathology, as diagnosed by visual assessment of MRI scans. The data are summarized in Table 2 and Table 3.
Neuropsychological Profile
There were no differences in performance IQ (Wechsler Adult Intelligence Scale—Revised) between psychotic and nonpsychotic patients. Verbal IQ was significantly lower in the psychotic group (mean verbal IQ: POE: 86.4, standard error [SE] 2.3; TLE control: 95.1, SE 3.0; T = 2.307, p = 0.02; mean performance IQ: POE: 89.7, SE 2.6; TLE control: 90.8, SE 4.7; T = 0.203, p = 0.8; mean total IQ: POE: 86.5, SE 2.3; TLE control: 93.6, SE 2.9; T = 1.902, p = 0.06; P-values not corrected for multiple comparisons).
Gray Matter Differences
Temporal lobe epilepsy and POE versus TLE alone—whole groups.
There were no significant differences with respect to increase or decrease of gray matter concentration between the whole group of patients with TLE and POE (n = 26) and the group with TLE alone (n =24).
Temporal lobe epilepsy and POE versus TLE alone—subgroups according to MR- and EEG-pathology.
There were no significant differences with regard to gray matter increase or decrease in the following subgroups: TLE and POE without MR lesions (n = 13) versus TLE alone without MR lesions (n = 7); TLE and POE with left-sided MR lesions (n = 6) versus TLE alone with left-sided MR lesions (n = 10); TLE and POE with left-sided MR or EEG pathology (n = 10) versus TLE alone with left-sided MR or EEG pathology (n = 12); and TLE and POE with right-sided MR or EEG pathology (n = 5) versus TLE alone with right-sided MR or EEG pathology (n = 10).
Temporal lobe epilepsy and POE versus TLE alone—whole groups with age, IQ, and amygdala, hippocampal, and total brain volume as covariates.
Volumes of amygdalae, hippocampi, and total brains in this sample were measured in a previous study.10 Using these variables, both separately and altogether as possible confounding covariates, several ANCOVAs produced no significant differences in decrease or increase of gray matter.
Schizophrenia-like psychosis versus PIP.
There was no significant difference in gray matter density between these groups.
Temporal lobe epilepsy alone versus healthy controls—whole groups.
The TLE alone group showed a significant increase in gray matter concentration in the right temporal lobe (Table 4).
DISCUSSION
In our study we investigated a possible role of differences in gray matter concentration in the pathogenesis of POE in patients with TLE. We did not find significant differences in gray matter concentration between patients with POE and TLE and those with TLE alone.
Methodological limitations of our study should be considered prior to an interpretation of our findings.
Methodological Issues
Patient selection.
First, our study is retrospective in nature. Thus, unfortunately, we were unable to obtain an elaborate psychometric assessment of study patients, retrospectively, since the patients were referred from various regions of the UK. All patients were seen and diagnosed by a neuropsychiatrist expert in epilepsy (MRT). All three study groups were matched for age and sex, and the two patient groups were matched regarding duration of epilepsy. Patients with any DSM-IV axis I disorder, except for psychosis of epilepsy, were excluded from further analysis. The dysphoric disorder of epilepsy26 was not excluded since this disorder is very common in epilepsy. Only patients with temporal lobe epilepsy were included in our study. All patients received a thorough neurological, psychiatric, and neuropsychological work up. Patients with extratemporal or generalised epilepsies were excluded, as were those with a full scale IQ ≤ 70. Thus, we were able to study a homogeneous, well defined, and well assessed sample of patients with POE that, to date, is the largest sample published in the literature. A disease control group with schizophrenia would have been desirable. However, this was impossible because there were no patients with schizophrenia at the Chalfont Centre for Epilepsy, and our plan to study patients from a distant psychiatric hospital proved to be unrealistic for different practical reasons.
Sample size and power.
Given our mainly negative findings, a larger sample size would have been preferable. However, the 3D datasets used in our study were acquired only since 1995, thus we could not recruit more patients from our center. Still, our sample of 26 patients with TLE and POE and 24 matched patient controls with TLE alone is, to our knowledge, the largest sample reported in the imaging literature. Furthermore, there are two reasons why, by inference, we consider our sample sufficiently large to detect differences between groups. First, several studies using a very similar methodology in SPM99 and smaller samples were able to detect significant differences in gray matter concentrations of patients with schizophrenia and healthy controls.27,28 Second, we were able to find significant differences in the temporal lobes between patients with TLE alone and healthy controls, which argues for the sensitivity of our analyses.
Motivation for our improved protocol.
In our study, we used a refined version of the conventional preprocessing protocol for voxel-based morphometry. Ashburner and Friston22 describe this conventional method, which consists of the three steps normalization, segmentation, and smoothing. This is a well-established process and has been successfully applied in numerous studies. To improve this method further, we included a step of brain extraction following the recommendation of Good et al.23 All images, including those used for the template, were acquired on the same scanner in order to avoid group differences for technical reasons.
Gray and White Matter Changes in POE
There have been early attempts to identify cortical changes in POE using CT scans that did not show enlarged cerebral sulci or other evidence of cortical damage.29 More recent MRI studies examined the cortex in POE, and there was no evidence of cortical changes.30 Consistent with these observations, we again did not find any evidence of gray matter abnormalities in our sample of patients with POE.
POE and Schizophrenia
We studied possible differences in gray matter concentration between patients with POE and TLE controls. Both groups share the temporal pathology involved in temporal lobe epilepsy. We did not find any evidence of cortical gray matter pathology in the POE group. Thus, unlike in schizophrenia, widespread cortical pathology apparently is not involved in the pathogenesis of POE. Instead, subcortical temporal lobe pathology involving limbic structures seems to be more relevant.10 Unfortunately, a schizophrenia control group was unavailable for reasons discussed above. However, among other studies mentioned before, Ananth et al.17 recently found widespread cortical changes in schizophrenia using the same VBM methodology that we used in a sample size of 20 patients with schizophrenia and healthy subjects. In contrast to the negative findings for gray matter changes, there have been repeated reports of white matter pathology in POE. Conlon et al. reported changes in the temporal white matter.30 Another neuropathologic study described perivascular white-matter softenings in schizophrenia-like psychosis, periventricular gliosis, and enlarged lateral ventricles that are commonly attributed to subcortical brain damage.31,32
Taken together, these studies support the notion that, unlike in schizophrenia, subcortical and, in particular, temporolimbic brain abnormalities are of importance in the pathophysiology of POE, while there is no evidence of cortical brain pathology.
CONCLUSIONS
Our findings support the notion that POE is a separate nosologic and pathological entity different from schizophrenia. From a morphological point of view, there are not only specific subcortical temporal lobe abnormalities in POE such as the amygdala enlargement reported previously,10 but also cortical abnormalities, previously described in schizophrenia patients and not present in POE. Furthermore, since there were no differences between postictal and interictal psychosis, as in our previous study, one might hypothesize that PIP and SLPE represent two different stages of one pathogenetic disorder, where SLPE possibly develops on the basis of PIP. This possibility suggests the importance of careful neuropsychiatric assessment and, perhaps, early and preventive treatment of the latter to avert the development of chronic psychosis in TLE.
ACKNOWLEDGMENTS
Dr. Rüsch is grateful for support by the Wissenschaftliche Gesellschaft, Freiburg, the Chalfont Centre for Epilepsy, and the Raymond Way Fund. This work would not have been possible without the methodological support in SPM and computing from Dr. M. Koepp and Dr. L. Lemieux, both at the Chalfont Centre for Epilepsy, and without the friendly and invaluable help of Heidi Gwynne, Dr. Afraim Haddadi, and Dr. Martin Merschemke during Dr. Rüsch's work at the Chalfont Centre for Epilepsy.
 |
 |
 |
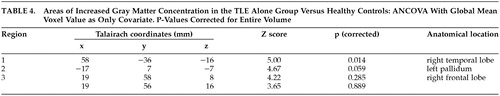 |
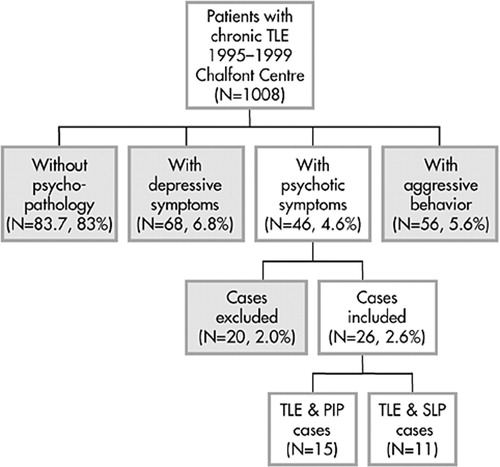
FIGURE 1. Illustration of the Selection Process
1 Gudmundsson G: Epilepsy in Iceland. A clinical and epidemiological investigation. Acta Neurologica Scandinavica 1966; 43:Suppl. 25, 113–124Google Scholar
2 Schmitz B, Wolf P: Psychosis in epilepsy: Frequency and risk factors. Journal of Epilepsy 1996; 8:17–25Google Scholar
3 Bredkjaer SR: Epilepsy and non-organic non-affective psychosis. National epidemiologic study. Br J Psychiatry 1998; 172:235–238Crossref, Medline, Google Scholar
4 Toone BK: The psychoses of epilepsy and the functional psychoses: a clinical and phenomenological comparison. British Journal of Psychiatry 1982; 141:256–261.Crossref, Medline, Google Scholar
5 Slater E, Beard AW: The schizophrenia-like psychosis of epilepsy. Br J Psychiatry 1963; 109:95–150Crossref, Medline, Google Scholar
6 Briellmann RS, Kalnins RM, Hopwood F, et al: TLE patients with postictal psychosis: Mesial dysplasia and anterior hippocampal preservation. Neurology 2000; 55:1027–1030Crossref, Medline, Google Scholar
7 Umbricht D: Postictal and chronic psychoses in patients with temporal lobe epilepsy. Am J Psychiatry 1995; 152:224–231Crossref, Medline, Google Scholar
8 Adachi N: Inter-ictal and post-ictal psychoses in frontal lobe epilepsy: a retrospective comparison with psychoses in temporal lobe epilepsy. Seizure 2000; 9:328–335Crossref, Medline, Google Scholar
9 Andermann LF: Psychosis after resection of ganglioglioma or DNET: evidence for an association. Epilepsia 1999; 40:83–87Crossref, Medline, Google Scholar
10 Tebartz van Elst L, Baeumer D, Lemieux L, et al: Amygdala abnormalities in psychosis of epilepsy. A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain 2002; 125:140–149Crossref, Medline, Google Scholar
11 Harrison PJ: The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 1999; 122:593–624Crossref, Medline, Google Scholar
12 Trimble MR, Ring HA, Schmitz B: Neuropsychiatric aspects of epilepsy. In Neuropsychiatry, edited by Fogel BS, Schiffer RB, Rao SM. Baltimore, Williams & Wilkins, 1996, pp 771–803Google Scholar
13 Pearlson GD: Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Progress in Neuro-Psychopharmacology & Biological Psychiatry 1997; 21:1203–1229Crossref, Medline, Google Scholar
14 Gur RE, Cowell PE, Latshaw A, et al.: Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 2000; 57:761–768Crossref, Medline, Google Scholar
15 Goldstein JM, Goodman JM, Seidman LJ, et al.: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Crossref, Medline, Google Scholar
16 Wilke M, Kaufmann C, Grabner A, et al: Gray matter changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. NeuroImage 2001; 13:814–824Crossref, Medline, Google Scholar
17 Ananth H, Popescu I, Critchley HD, et al: Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 2002; 159:1497–1505Crossref, Medline, Google Scholar
18 Hulshoff Pol HE, Schnack HG, Mandl RCW, et al.: Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry 2001; 58:1118–1125.Crossref, Medline, Google Scholar
19 Logsdail SJ, Toone BK: Post-ictal psychoses. A clinical and phenomenological description. Br J Psychiatry 1988; 152:246–252Crossref, Medline, Google Scholar
20 Ashburner J, Neelin P, Collins DL, et al: Incorporating prior knowledge into image registration. NeuroImage 1997; 6:344–352Crossref, Medline, Google Scholar
21 Ashburner J, Friston KJ: Nonlinear spatial normalization using basis functions. Human Brain Mapping 1999; 7:254–266Crossref, Medline, Google Scholar
22 Ashburner J, Friston KJ: Voxel-based morphometry—the methods. NeuroImage 2000; 11:805–821.Crossref, Medline, Google Scholar
23 Good CD, Johnsrude IS, Ashburner J, et al: A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001; 14:21–36Crossref, Medline, Google Scholar
24 Friston KJ, Holmes AP, Worsley K, et al: Statistic parametric maps in functional imaging: A general linear approach. Human Brain Mapping 1995; 2:189–210Crossref, Google Scholar
25 Worsley K, Marrett SN, Vandal AC, et al: A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping 1996; 4:58–73Crossref, Medline, Google Scholar
26 Blumer D: Dysphoric disorders and paroxysmal affects: recognition and treatment of epilepsy-related psychiatric disorders. Harvard Review of Psychiatry 2000; 8:8–17.Crossref, Medline, Google Scholar
27 Paillere-Martinot M, Caclin A, Artiges E, et al.: Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophrenia Research 2001; 50:19–26Crossref, Medline, Google Scholar
28 Sowell ER, Levitt J, Thompson PM et al.: Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am J Psychiatry 2000; 157:1475–1484Crossref, Medline, Google Scholar
29 Perez MM, Trimble MR, Murray NMF, et al: Epileptic psychosis: An evaluation of PSE profiles. Br J Psychiatry 1985; 146:155–163Crossref, Medline, Google Scholar
30 Conlon P, Trimble MR, Rogers D: A study of epileptic psychosis using magnetic resonance imaging. Br J Psychiatry 1990; 156: 231–235Google Scholar
31 Kristensen O, Sindrup EH: Psychomotor epilepsy and psychosis. Acta Neurologica Scandinavica 1978; 57:361–379Crossref, Medline, Google Scholar
32 Stevens JR: Epilepsy and psychosis: neuropathologic studies of six cases, in Aspects of epilepsy and psychiatry, edited by Bolwig T, Trimble MR. London, John Wiley & Sons, 1986, pp 117–146Google Scholar



