Twenty-Four-Hour Rhythms of Sleep-Wake Cycle and Temperature in Alzheimer's Disease
Abstract
The authors aimed to examine the difference in 24-hour rhythms of sleep-wake cycle and temperature between Alzheimer's disease (AD) patients and elderly comparison subjects. The continuous measuring of wrist activity and skin temperature was conducted for 96 hours in seven AD patients (age: 77.0±4.3) and 11 normal comparison subjects (age: 74.2±5.2). The mean acrophases and amplitudes of the two rhythms in the AD group were not different from those in the comparison group. The mean phase difference between the two rhythms, however, was significantly lower in the AD group than in the comparison group.
Dysfunction of the circadian timing system has long been suspected in Alzheimer's disease (AD) because such patients frequently have sleep disturbance.1–3 A limited number of studies2,4–6 suggest that agitated behaviors in demented elderly patients, with the peak of agitation occurring just after sunset, which has been called “sundowning,” are associated with the underlying circadian rhythm. Despite such sleep disruption, some studies of various physiological phenomena, typically showing diurnal variation, have difficulty revealing differences between AD patients and controls.7,8 Sleep disturbance, which could affect the maintenance of mood, alertness, and cognitive performance during the day, causes further clinical impairment in dementia patients and greater caregivers' burden.9 This problem usually results in the institutionalization of dementia patients, contributing to the continued disruption of circadian rhythms.10
The suprachiasmatic nucleus in anterior hypothalamus, which was indicated as the circadian clock in previous studies, has shown significant degeneration of the vasopressin-containing neuron in aging and AD.7,11 This may be related to the change of circadian rhythm in aging, possibly explaining the sleep disturbance in the elderly.12–15
It has been shown that age-related changes in circadian sleep wake-organization are accentuated in AD.16,17 However, studies of body temperature rhythm have not supported a parallel decrease in rhythm amplitude.2,18 The changes of circadian rhythm in aging did not show consistent results in the previous studies. The sleep-wake rhythm becomes polyphasic with aging due to fragmentation of nocturnal sleep and increased napping during the day. Additionally, the amplitude reduced along with decreased activity in the elderly.19–21 Amplitude of temperature rhythm decreased with aging in the entrained condition, but not in the free-running condition.12,13
In our study, we aimed to assess whether there are any differences in circadian rhythms of sleep-wake cycle and temperature between AD patients and normal aged controls in the entrained condition. There are a limited number of studies regarding dementia patients residing at their homes (in their natural environments) rather than in nursing homes. We also analyzed the phase relationship between the two rhythms in the entrained condition in order to find any change in the circadian organization in elderly patients with AD.
METHODS
Seven patients with AD (age: 77.0±4.3, three men and four women) and 11 normal comparison subjects (age: 74.2±5.2, four men and seven women) who were registered with the Alzheimer Center at Fairhill Institute for the Elderly, affiliated with the University Hospitals of Cleveland, participated in this study. The mean age in the AD group was not significantly different from that of the comparison group. The diagnosis of AD was made according to the NINCDS-ADRDA criteria using the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Assessment Packet.22 On the Clinical Dementia Rating (CDR) Scale,23 AD patients received a score of 1 or 2. Subjects who had known psychiatric disorders or were taking any psychotropic drugs during the study were excluded. All subjects were free of investigation drug use at least 1 month prior to entrance into the study. All comparison subjects were evaluated by the CERAD Assessment Packet and found not to have cognitive decline, psychiatric disorders, or physical disorders.
Prior to measuring the sleep-wake and temperature rhythms, a sleep-log diary was given to each subject. Continuous measuring of wrist activity and skin temperature on the axillary region was conducted by a Mini-Logger™ ambulatory monitoring device (Mini-Mitter Company, Oregon, USA) for 96 hours (4 consecutive days and nights), starting at 8 a.m. on the first day of monitoring for each subject. Previous validation studies indicated that agreement rates between actigraph-based and polysomnograph (PSG)-based minute-by-minute sleep-wake scoring were very promising for normal subjects (above 90%).24 The ability to document rest-activity circadian rhythmicity from actigraphic data has been well demonstrated in several studies.25–27 Subjects were allowed to perform routine activities as usual during the monitoring period and asked to remove the actiwatch and skin temperature probe only while showering. An activity log was kept during the ambulatory monitoring in order to record sleep-wake cycles and the times in which the monitoring device was removed. Informed consent was obtained after the procedure had been fully explained.
Every minute of wrist activity was scored as either wake or sleep, as described by Cole et al.28 Every 16-minute interval was labeled as wake if 8 or more minutes were scored as wake and as sleep if 9 or more minutes were scored as sleep. The mean temperature was calculated for the 16-minute interval.
For the statistical analysis of the sleep-wake and temperature rhythms of each individual, a cosinor analysis was applied to calculate the circadian rhythm measures: acrophase (phase angle of the maximum value of a sine function fitted to the raw data of a rhythm) and amplitude (difference between maximum and mean value in a sinusoidal oscillation.29 Since the raw data of wrist activity were presented as 0 or 1 (wake or sleep), a noncontinuous variable, the moving average technique was added. We called it Model 1, which has the following functional formula for curve fitting of the 24-hour rhythm.

Since we found that the cosine curve (dotted line) by the Model 1 analysis was not well suited to our actual data of the 24-hour sleep-wake rhythms (Figure 1), we applied the Model 2 analysis, which has the formula below. The actual line by Model 2 analysis was different from the dotted one by Model 1 analysis.
We also used the Model 1 analysis for the temperature rhythm (Figure 2). For each individual, curve fittings of both wrist activity and skin temperature measures were conducted from the data averaged with respect to the time of day. To compare the mean acrophase and amplitude of the AD group with those of the normal group, Student's t test was performed. We determined the phase difference between the sleep-wake rhythm and temperature rhythm in each subject and compared the means of the AD and comparison groups. Two comparison subjects, whose data of wrist activity were mostly 0, were excluded from the analysis for the sleep-wake rhythm because curve fittings could not be done for them.
RESULTS
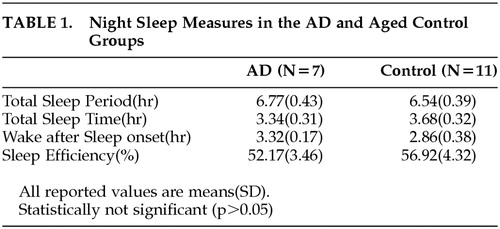
Table 1 shows the comparison of the night sleep measures analyzed from the rest-activity data between the AD group and the comparison group. The sleep measure of each individual was calculated for each day, and the average of these values for 4 days in each individual was used for obtaining the means of both groups. There was no statistically significant difference in the sleep measures (e.g., total sleep period, total sleep time, wake-after-sleep onset, sleep efficiency) between the two groups (p > 0.05), although the mean of sleep efficiency was slightly lower, and the mean of wake after sleep onset slightly greater, in AD patients relative to those in comparison subjects.
The difference in the mean acrophases of the temperature rhythm between the AD group and the comparison group was almost 3 hours (Table 2). However, it was not statistically significant (p = 0.05). Additionally, there was no difference in the mean amplitude between the two groups. Table 3 shows the mean acrophases and amplitudes of the 24-hour rhythm of the sleep-wake cycle in both groups, which were the results taken from the Model 1 and Model 2 analyses. There was about 1-hour difference between the mean acrophases of the AD group and the comparison group. This result was similar to that taken from the Model 2 analysis. However, no significant difference in the mean acrophases between the two groups was found (p > 0.05). There was also no significant difference in the mean amplitude of the sleep-wake rhythm between the AD and comparison groups, according to both Model 1 and Model 2 analyses, although the absolute values of the two groups in Model 1 were different from those in Model 2.
The phase difference, the difference in the acrophases between the sleep-wake rhythm and temperature rhythm was significantly lower in the AD group than in the comparison group. This shortening of the mean phase difference in the AD group appears to be attributed to the phase delay in the temperature rhythm (Table 2) and the phase advance in the sleep-wake rhythm (Table 3), when compared to the rhythms in the normal group. Additionally, the phase relationship between the two rhythms in the AD group by the Model I analysis showed a reverse relationship, in which the acrophase of the sleep-wake rhythm was earlier than in the temperature rhythm. The phase relationship by the Model 2 analysis, however, showed only the shortening of the phase difference between the two rhythms, keeping the same trend in the phase relationship.
DISCUSSION
Our results showed that there were no differences in the acrophases and amplitudes of the 24-hour sleep-wake and temperature rhythms between the AD and elderly comparison groups. Previous studies regarding the circadian rhythm in aging and dementia have suggested that the real manifestation by the circadian oscillators would be masked in the entrained condition. The decrements of the circadian period and amplitude of the body temperature rhythm with aging, which had been found in the entrained condition, were not observed in the free-running stage. However, in the experimental condition of constant routine, which unmasks the endogenous circadian oscillator, the circadian period and the phase relationship with the zeitgeber in the temperature rhythm was shortened in aging.12,30 This finding could be associated with the phase advance of body temperature rhythm in the elderly, resulting in the internal desynchronization. The amplitude reduction of the circadian temperature regarding the sleep consolidation was also noted in individuals in the healthy aged group who experienced a 6-hour phase advance in constant routine.13
Our study was conducted in the usual entrained condition for the subjects who were residing at home and continuing their routine daily activities, while monitoring the sleep-wake and temperature rhythms. In fact, most of the previous studies on circadian rhythm in dementia patients were done in their nursing home environment, with the exception of a few studies.21,31 In our study, the clinical severity of AD patients was mild or moderate (CDR: 1 or 2), while AD patients seemed to have higher CDR scores in the previous studies of Satlin et al.32 and Bliwise at al.2 Mean MMSE scores (SD) of the subjects were 0.6 (1.1) and 8.9 (4.7), respectively. No significant difference in the values of circadian parameters between the AD and elderly comparison groups could be explained by a relatively less severe clinical state of dementia patients in our study. It has been reported that no change in the amplitude of sleep-wake cycle was observed in mild AD patients.33 Witting et al.34 also reported that the sleep-wake rhythm is disturbed in AD patients and correlates with the severity of dementia. To the contrary, a recent study didn't show any correlation between the two.10 In our study, the number of AD patients was too small to conduct the correlation analysis between the amplitude of sleep-wake rhythm and the severity of dementia.
Both the skin temperature and sleep-wake rhythms are regulated by the “Y” pacemaker, which is a labile and weak circadian oscillator, while the core body temperature rhythm by the “X” pacemaker is stable and strong.35 Therefore, the phase difference in our result has a different meaning from the phase relationship between the two major circadian oscillators, “X” and “Y.” In other words, the significant shortening of the phase difference between the two rhythms in our study does not actually indicate an internal desynchronization, which has been suggested as a possible biological marker of AD.36 However, the change in the phase relationship between two different rhythms in AD patients, relative to that of the elderly comparison subjects, suggests that the stability of their relationship was broken to some degree. However, it will be difficult to exclude a false negative possibility of our finding because of its small sample size.
It has been noted that the sundowning syndrome in AD was closely related with the sleep-wake rhythm disturbance.2,32 Our study showed no significant change in the absolute values of the circadian variables, but with the change in the circadian organization in AD patients relative to elderly comparison subjects, we could still address the significance of the phase difference in our study since it was shown even in AD patients without serious sleep or behavioral disturbances such as sundowning syndrome. As illustrated in Figure 2, the variability in the skin temperature profile for 24 hours was remarkably higher, as compared to the core body temperature profile shown in previous studies.37,38 However, skin temperature still showed a clear circadian organization in our study and would reflect change in the phase relationship with the sleep-wake rhythm. Furthermore, most of the subjects felt relatively comfortable with the skin temperature monitoring in our study.
In our study, the statistical analysis was conducted based on the frequently used method in previous studies on circadian rhythms. However, a slightly different analysis was added to a simple cosinor analysis of the sleep-wake rhythm. The Model 1 analysis did not show any significantly different result from that of the Model 2 analysis, except the reversed phase relationship between the two rhythms in the AD group. In the case of showing a distinct circadian organization, applying an alternative way of analysis for a certain circadian rhythm would be required.
 |
 |
 |
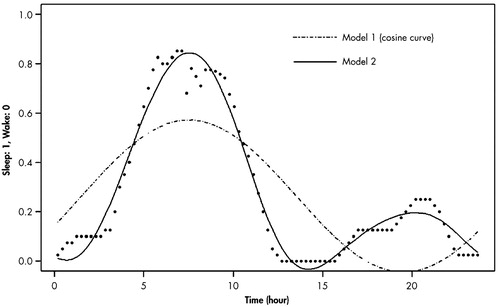
FIGURE 1. Curve Fitting of the Wrist Activity Measures Averaged With Respect to Time of Day for a Patient With Alzheimer’s Disease by Model I and II Analyses
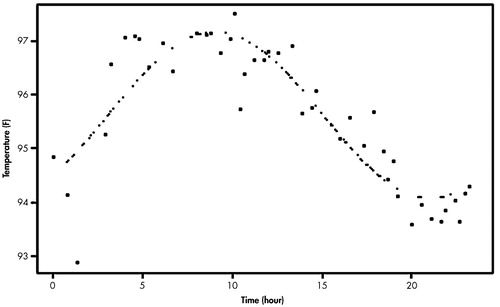
FIGURE 2. Curve Fitting of the Skin Temperature Measures Averaged With Respect to Time of Day for a Patient With Alzheimer’s Disease
1 Reynolds CF, Hoch CC, Stack J, et al.: The nature and management of sleep/wake disturbance in Alzheimer’s dementia. Psychopharm Bulletin 1988; 24:43–48Medline, Google Scholar
2 Bliwise DL, Carroll JS, Lee KA, et al.: Sleep and “sundowning” in nursing home patients with dementia. Psychiatry Res 1993; 48:277–292Crossref, Medline, Google Scholar
3 Cohen-Mansfield J, Werner P, Freedman L: sleep and agitation in agitated nursing home residents: an observational study. Sleep 1995; 18(8):674–680Medline, Google Scholar
4 Cohen-Mansfield J, Werner P, Marx MS: Screaming in nursing home residents. J Am Geriat Soc 1990; 38:785–792Crossref, Medline, Google Scholar
5 Martino-Saltzman D, Blasch BB, Morris RD, et al.: Travel behavior of nursing home residents perceived as wanderers and non-wanderers. The Gerontologist 1991; 31:666–672Crossref, Medline, Google Scholar
6 Martin J, Pat-Horenczyk R, Corey-Bloom J, et al.: Circadian patterns of agitation in demented nursing home patients. Sleep Res 1997; 26:163Google Scholar
7 Prinz PN, Christie C, Smallwood R, et al.: Circadian temperature variation in healthy aged and in Alzheimer's disease. J Gerontol 1984; 39:30–35Crossref, Medline, Google Scholar
8 Satlin A, Teicher MH, Lieberman HR, et al.: Circadian locomotor activity rhythms in Alzheimer's disease. Neuropsychopharmacology 1991; 5:115–126Medline, Google Scholar
9 Carskadon MA, Brown ED, Dement WC: Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging 1982; 3:321–327Crossref, Medline, Google Scholar
10 Ancoli-Israel S, Klauber MR, Jones DW, et al.: Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing home patients. Sleep 1997; 20(1):18–23Medline, Google Scholar
11 Swaab DF, Fliers E, Partiman TS: The suprachiasmatic nucleus in the human brain in relation to sex, age and senile dementia. Brain Res 1985; 342:37–44.Crossref, Medline, Google Scholar
12 Myers BL, Badia P: Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci and Biobehavioral Rev 1995; 19(4):553–571Crossref, Medline, Google Scholar
13 Carrier J, Monk TH, Buysse DJ, et al.: Amplitude reduction of the circadian temperature and sleep rhythms in the elderly. Chronobiol International 1996; 13(5):373–86Crossref, Medline, Google Scholar
14 Gigli GL, Placidi F, Diomedi M, et al.: Sleep in healthy elderly subjects: A 24-hour ambulatory polysomnographic study. Intern J Neuroscience 1996; 85:263–271Crossref, Medline, Google Scholar
15 Kayukawa Y, Kogawa S, Tadano F, et al.: Sleep problems in the aged in relation to senility. Psychiatry and Clin Neurosci 1998; 52(2):190–192Crossref, Medline, Google Scholar
16 Van Someren EJW, Kessler A, Mirmiran M, et al.: Indirect bright light improves circadian rest-activity rhythm disturbances in dementia patients. Biol Psychiatry 1997; 41:955–963Crossref, Medline, Google Scholar
17 Pat-Horenczyk R, Klauber MR, Shochat T, et al.: Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging 1998; 10(4):308–15Medline, Google Scholar
18 Touitou Y, Reinberg A, Bordan A, et al.: Age-related changes in both circadian and seasonal rhythms of rectal temperature with special reference to senile dementia of Alzheimer type. Gerontology 1986; 32:110–118Crossref, Medline, Google Scholar
19 Haimov I, Lavie P: Circadian characteristics of sleep propensity function in healthy elderly: a comparison with young adults. J of Biol Rhythms 1997; 12(6):627–635Crossref, Medline, Google Scholar
20 Kramer CJ, Kerkhof GA, Hofman WF: Sleep-wake behavior and circadian rhythmicity in aging. Sleep Res 1997; 26:717Google Scholar
21 Sakurai N, Sasaki M: An activity monitor study on the sleep-wake rhythm of healthy aged people residing in their homes. Psychiatry and Clin Neurosci 1998; 52(2):253–255Crossref, Medline, Google Scholar
22 Morris JC, Heyman A, Mohs RC, et al.: The Consortium to Establish a Registry for Alzheimer's Disease (CERAD)-Part 1. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39:1159–1165Crossref, Medline, Google Scholar
23 Hughes C, Berg L, Danziger WL, et al: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Crossref, Medline, Google Scholar
24 Sadeh A, Hauri PJ, Kripke DF, et al: The role of actigraph in the evaluation of sleep disorders. Sleep 1995; 18(4):288–302Crossref, Medline, Google Scholar
25 Lieberman HR, Wurtman JJ, Teicher MH. Circadian rhythms of activity in healthy young and elderly humans. Neurobiol Aging 1989; 10:259–265Crossref, Medline, Google Scholar
26 Brown AC, Smolensky MH, D'Alonzo GE, et al: Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int 1990; 7:125–133Crossref, Medline, Google Scholar
27 Tzichinsky O, Haimov I, Lavie P. Analysis of actigraphic records-searching for an optimal method. Sleep Res 1996; 25:535Google Scholar
28 Cole RJ, Kripke DF, Gruen W, et al: Automatic sleep/wake identification from wrist activity. Sleep 1992; 15(5):461–469Crossref, Medline, Google Scholar
29 Teicher MH, Lawrence JM, Barber NI, et al: Increased activity and phase delay in circadian motility rhythms in geriatric depression. Arch Gen Psychiatry 1988; 45:913–917Crossref, Medline, Google Scholar
30 Richardson GS (ed): Circadian rhythms and aging, in Handbook of the biology of aging, Third Edition, Academic Press, 1990, pp 275–305Google Scholar
31 Bliwise DL, Tinklenberg JR, Yesavage JA: Timing of sleep and wakefulness in Alzheimer's disease patients residing at home. Biol Psychiatry 1992; 31:1163–1165Crossref, Medline, Google Scholar
32 Satlin A, Volicer L, Ross V, et al: Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's disease. Am J Psychiat 1992; 149(8):1028–1032Crossref, Medline, Google Scholar
33 Aharon-Peretz J, Masiah A, Pillar T, et al: Sleep-wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology 1991; 41:1616–1619Crossref, Medline, Google Scholar
34 Witting W, Kwa IH, Eikelenboom P, et al: Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry 1990; 27:563–72Crossref, Medline, Google Scholar
35 Moore-Ede MC, Sulzman FM, Fuller CA (eds): The human circadian timing system, in The cloaks that time us, Cambridge and London, Harvard University Press, 1982, pp 295–317Google Scholar
36 Penev PD, Kolker DE, Zee PC, et al: Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. The Am Physiol Soc 1998; H2334-H2337Google Scholar
37 Duffy JF, Dijik DJ, Klerman EB, et al: Later endogenous circadian temperature nadir relative to an earlier wake time in older people. The Am Physiol Soc 1998; R478-R1487Google Scholar
38 Eastman CI, Liu L, Fogg LF: Circadian rhythm adaptation to simulated night shift work: Effect of nocturnal bright-light duration. Sleep 1995; 18(6):399–407Crossref, Medline, Google Scholar



