Toward a Neurobiology of Psychotherapy: Basic Science and Clinical Applications
Abstract
Psychotherapy is used commonly to treat a variety of mental illnesses, yet surprisingly little is known about its biological mechanisms especially in comparison with pharmacotherapy. In this review we survey the current knowledge about changes in brain function following psychotherapeutic intervention that are detectable with current neuroimaging techniques. We also consider the possible role for neuroimaging in refining clinical diagnoses and predicting treatment outcome, which would benefit both clinical decision-making and the cognitive neuroscience of psychotherapy.
Neuroscience has developed a number of methods for analyzing cognitive function that have enriched our understanding of normal and abnormal mental function. These insights also have improved the ability to intervene pharmacotherapeutically in the treatment of patients with mental illness. Can this understanding also inform psychotherapeutic interventions?
Despite dramatic advances in psychopharmacology, over the last few decades psychotherapy has continued to play an important role in the treatment of mental illness. Two forms of psychotherapy, cognitive-behavioral therapy and interpersonal therapy, have now been shown to be therapeutically effective in controlled clinical trials. Moreover, for certain disorders, such as major depression, the combined use of psychotherapy and medication can lead to better treatment outcomes than the use of either mode of therapy alone.1–3 Even in severe disorders like schziophrenia, certain types of psychotherapy, such as psychoeducation or cognitive behavior therapy, can enhance compliance with medication and reduce the frequency of hospitalization, even though these therapies do not directly affect the course of the disease.4 However, despite the extensive use of psychotherapy for a number of mental disorders, a biological perspective on its mechanisms remains to be defined.
The need for a biological perspective is self-evident. Psychopharmacological research continues to lead to the development of more specific and effective medications with increasingly benign side effects. We would argue that research is similarly needed into the mechanisms of psychotherapeutic action on the biological, cognitive, and behavioral levels, yet psychotherapy research lags far behind that on pharmacotherapy. This is in part due to the cost, inconvenience and difficulty of conducting and evaluating a complete course of psychotherapy under controlled conditions. Until the last decade, the biological mechanisms of psychotherapeutic actions were thought not to be amenable to neurobiological investigation. With the advent of neuroimaging techniques with high spatial and temporal resolution, the ability to probe the biological consequences of psychotherapeutic interventions has begun to come within reach, and with it the ability to document psychotherapy’s effectiveness, to follow its course, and to refine its appropriate applications for selected patients and disorders. In addition to the practical indications, investigating the biology of psychotherapy is also important for psychiatry’s attempt to link specific mental functions with specific brain mechanisms. This understanding may also aid in the analysis of how the environment affects the brain. Psychotherapy is a controlled form of learning that occurs in the context of a therapeutic relationship. From this perspective, the biology of psychotherapy can be understood as a special case of the biology of learning.5
Because the neurobiological study of psychotherapy is in its infancy, our attempt to analyze these studies at this early point is of necessity incomplete. Nonetheless, even this initial foray provides several new initial insights into what will be feasible in the near future. We therefore outline not only where we are now, but also how future refinements might advance the field. We limit our discussion to three disorders where neuroimaging work is most advanced: depression, obsessive-compulsive disorder (OCD), and anxiety states. In considering these illnesses, we focus on the use of neuroimaging for diagnosing and understanding psychopathology, for following the course of the disorder, and for predicting outcome of treatment.
Basal Brain Imaging: Detecting Changes Associated With Psychotherapy
Most neuroimaging studies of psychotherapy have focused on depression and OCD and have examined basal brain metabolism or basal cerebral blood flow in these disorders.6–10 The several published studies are consistent in demonstrating changes following psychotherapy in brain activity in patients with these disorders when compared with healthy comparison subjects. Successful treatment frequently restored the brain to a state that superficially resembled that of comparison subjects. Particularly interesting is the finding that some of the changes accompanying successful psychotherapy resembled those seen with pharmacotherapy, with the suggestion that, at least in some cases, psychotherapy and medications may act on a common set of brain targets.
The early neuroimaging studies of OCD used fluorodeoxyglucose–positron emission tomography (FDG-PET). A typical scan involves continuous acquisition for 30–40 minutes while patients are at rest, and these scans therefore were not sensitive to moment-to-moment changes in neuronal activity, as might happen during performance of a cognitive task. In the first such study, Baxter et al.6 found an increase in basal glucose metabolism in the caudate nucleus of OCD patients. Treatment with either the selective serotonin reuptake inhibitor (SSRI) fluoxetine or exposure psychotherapy reversed the metabolic abnormality associated with the disorder. A second study of another population found that patients who responded to psychotherapy showed greater decreases in right caudate metabolism than patients who did not respond.10 Although both initial studies lacked important controls, they nonetheless demonstrated that psychotherapy could produce a detectable change in brain activity.
Subsequent FDG-PET studies of psychotherapy have focused primarily on depression. The most common FDG-PET finding in depression is a decrease in the basal activity of the dorsolateral prefrontal cortex. Less consistently reported is increased activity in the ventrolateral prefrontal cortex.11–14 Both SSRIs and electroconvulsive therapy reversed these abnormalities.11 To relate these findings to psychotherapy, two studies compared interpersonal psychotherapy with either the SSRI paroxetine or the serotonin-norepinephrine reuptake inhibitor venlafaxine in the treatment of depression.8,9 Again, psychotherapy reversed pretreatment abnormalities, including those in the prefrontal cortex, similar to the effects of pharmacotherapy.
The conclusion from these two sets of studies is that psychotherapy is similar to pharmacotherapy in normalizing functional abnormalities in brain circuits that give rise to symptoms. It is likely, however, that this view is incomplete. With further research it should be possible to distinguish common from distinct changes among these very different forms of therapy. This may allow one to distinguish between brain regions that contribute to symptom improvement per se and those that contribute to the mechanisms of a particular therapy. For example, Goldapple et al.15 found that depressed patients treated with cognitive-behavioral therapy show some common and some different brain changes when compared with patients treated with paroxetine. Thus, the initial idea in the literature that psychotherapy and pharmacotherapy produce similar changes is not likely to prove generally true. Indeed, firm conclusions cannot be drawn from these early studies, because they are hampered by lack of or incomplete randomization for treatment types, as well as by lacking certain controls.
Moving Beyond Basal Brain Function: Stimulus-Responsive Imaging of the Effects of Psychotherapeutic Interventions
Recent studies have sought to go beyond the measurement of basal metabolism by examining the effect of psychotherapy on context-specific neural responses in disease-relevant tasks.16,17 In one such study, Furmark et al.16 (using PET measures of changes in regional blood flow secondary to neuronal activation) examined patients with social phobia treated with either citalopram or cognitive-behavioral group therapy. Tillfors et al. had previously found that when patients with social phobia gave a prepared speech in the scanner in the presence of others, they had a larger increase in regional blood flow in the amygdala and hippocampus, compared with comparison subjects (as compared with giving the speech alone).18 Improvement in symptoms with treatment was accompanied by decreased activity in the amygdale, and the medial temporal lobe in the stressful public speaking condition (Figure 1). No such changes were seen in waiting-list comparison subjects. Comparing treatment groups with a comparison group of waiting-list patients who received no treatment allowed Furmark et al. to rule out changes related only to the rescanning of subjects or simply to the passage of time. Decreases in the activity of the amygdala were seen in both the cognitive-behavioral therapy and the citalopram groups. The two treatment groups, however, differed with respect to changes outside the amygdala. Interestingly, the degree to which amygdala activity decreased as a result of therapy predicted the reduction in patients’ symptoms one year later. Unfortunately, because of small sample sizes, the study was not able to distinguish between responders and nonresponders for each modality. This distinction might have allowed a dissociation of the symptom-improving effects of the treatment from the consequences of having received the treatment per se.
Despite their limitations, both the studies of basal metabolism and those of stimulus-responsive imaging have demonstrated that psychotherapy produces changes in the brain, some of which may be shared with those induced by pharmacotherapy, whereas others may be modality-specific. As the neurobiological substrates of psychotherapeutic change are better defined, more directed animal studies can also be focused on those brain regions and the functional networks in which they participate.
Future Advances in the Neuroimaging of Psychotherapy
Neuroimaging can be a highly sensitive mode of investigation. Rather than looking at a single dependent measure (e.g., reaction times), neuroimaging simultaneously assesses the activity of every part of the brain. This flexibility can be enhanced by use of a variety of stimuli and tasks, as well as data analytic techniques that can separately probe the data for activations, connectivity and network-level interactions.
This sensitivity of neuroimaging has several implications. First, neuroimaging may provide an independent way of grouping patients based on specific biological variables, that are closer to aspects of the pathogenesis of the disease. Major depression, for example, is likely to have multiple distinct etiologies, and these distinct etiologies may be clinically indistinguishable. Patient subgrouping may reveal why some patients improve with particular therapies and others do not.
The power of novel data analysis techniques for objectively subgrouping subjects on the basis of biological criteria was illustrated by Meyer-Lindenberg et al.19 They used multivariate analysis and found that the expression of a brain-wide pattern of activity in an individual almost perfectly separated a group of schizophrenic patients from a comparison group (like a diagnostic marker). In this way they were able to use resting brain scans to separate out two independent cohorts with 94% accuracy. Most neuroimaging studies use univariate analysis methods and examine the activity of single brain regions at a time rather than groups of areas across the whole brain. This can result in a great degree of overlap between regional activation in a disease group and in a comparison group—a distinction that may otherwise be easily made by multivariate analysis methods.
Predictions of whether a particular therapy will work for a given patient may, instead, depend more on the functional characteristics of that individual’s brain than on how the patient is diagnosed. In other words, understanding how an individual’s brain processes particular stimuli may provide critical information for predicting treatment outcome. Neuroimaging-based quantification of regional brain function during disease-relevant and irrelevant tasks in a standardized way across patients may bring out many important differences. These differences may predict how a patient will process and respond to stimuli in the context of particular forms of psychotherapy, or after pharmacological treatment. Such an approach can be thought of as a “cognitive-emotional stress test,” much as cardiologists use exercise stress tests in conjunction with radionuclide scanning to bring out subtle changes in cardiac function that can predict disease progression and select the most indicated treatment from the range of medical and surgical options. Additionally, as in cardiology, serial scans of individual patients may permit the progress of treatment to be monitored more directly and precisely than ever before, and provide early signs of recovery or relapse well before changes in behavior or symptom ratings can be seen. Prediction of outcome may be successful even without a full understanding of why a particular pattern of brain activation predicts better outcome.
These arguments are based on the assumption that biological variables cause the behavioral manifestations of psychiatric disorders and therefore are likely to be more sensitive indices of underlying pathology than monitoring of symptoms. Moreover, neuroimaging measures are also sensitive to processes at the conscious and unconscious levels, as they must both be reflected by underlying processes in the brain. In addition, neuroimaging approaches might equally be used to help conceptualize the psychopathology and psychotherapy in functional terms.
Predicting Outcome With Neuroimaging
The most convincing outcome predictions come from neuroimaging studies of depression. While these studies relate to the pharmacotherapy of depression, they can be at least conceptually extended to psychotherapy. A landmark FDG-PET study of the pharmacological treatment of unipolar depression found that activity in the rostral anterior cingulate cortex (ACC) uniquely differentiated treatment responders from nonresponders.20 Responders were hypermetabolic prior to treatment with respect to comparison subjects, while nonresponders were hypometabolic (Figure 2A). The predictive value of pretreatment activity in the rostral ACC in depression has been confirmed by subsequent studies, as detailed below. Rostral ACC activity predicted better response to paroxetine treatment21 as well as to partial sleep deprivation therapy (Figure 2B).22,23 More recently, Pizzagalli et al.24 recorded scalp EEG activity in nortriptyline-treated depressed patients, focusing on one EEG frequency band thought to be generated by the anterior cingulate. Again, patients showing electrical hyperactivity in the rostral cingulate before treatment showed better response 4–6 months after treatment, an effect that was not related to pretreatment depression severity (Figure 2C). In the first study to examine functional recruitment of the rostral cingulate, rather than its baseline activity or metabolism, Davidson et al.25 examined functional magnetic resonance imaging (fMRI) activation in response to viewing negatively valenced visual stimuli, compared with neutral stimuli. They likewise found that higher pretreatment activation of the rostral cingulate predicted a lower depression symptom scale score 8 weeks after treatment (Figure 2D).
The anterior cingulate can be divided into three subdivisions (dorsal, ventral and rostral) by anatomical and functional imaging criteria.26,27 The dorsal (cognitive) division of the ACC is often activated by nonemotional tasks that produce conflicts between potential responses (i.e., a color-word Stroop Task in which the font color of the word to be identified is incongruent with the word itself: “red” in green ink). The ventral (affective) division of the ACC can be recruited by mood induction protocols.14,26 In depression, changes in both the dorsal and ventral regions of the cingulate have been seen.13,28,29 The rostral division of the ACC is located between these other two subdivisions. It receives input from both of the other divisions of the ACC and is thought to integrate them.27 Thus, the rostral ACC may be important for detecting conflict in the emotional domain and recruiting cognitive-attentional processes to resolve the conflict. In support of this view, the rostral ACC becomes more active when subjects are asked to ignore emotional words in a word counting task than when they are instructed to ignore neutral words.30 In this task, ignoring emotional content would be expected to increase conflict in the emotional domain.
One way to understand the potential role of the rostral ACC in conflict resolution and cognitive control is by examining the functions of the dorsal ACC under conflict conditions. Kerns et al.31 found that the dorsal division of the ACC was activated by conflict in a color-word Stroop task. Dorsal ACC activation by this conflict predicted greater recruitment of the dorsolateral prefrontal cortex on the subsequent trial. This increase in cognitive control decreased the incongruency effect, thereby leading to shorter reaction times for incongruent trials that were preceded by an incongruent trial than for incongruent trials that were preceded by a congruent trial. The dorsal ACC appeared to both resolve cognitive conflict and prime cognitive systems to better reduce conflict on the subsequent trial. By analogy, the rostral ACC may be recruited to resolve conflict between emotional stimuli or between conflicting mental content. In major depression, cognitive control may be important for regulating the effects of negative mood over perception, thoughts, and behavior. Patients with higher levels of activity in the rostral cingulate before therapy may thereby be in a better position for recovery.
Preliminary imaging studies of OCD implicate a different area of the prefrontal cortex, the orbitofrontal cortex (OFC), in predicting treatment response. The OFC is highly activated during symptom provocation in OCD patients.32,33 One FDG-PET study of OCD found that lower pretreatment levels of activity predicted better response to drug therapy (Figure 3A).34 More intriguing was the finding that lower pretreatment metabolism of the OFC predicted better response to medications than to psychotherapy, whereas higher OFC metabolism predicted the opposite7 (Figure 3B). As in many of the other studies cited earlier, several major caveats must be considered. Subjects were not randomized, blinded, or compared with a placebo group. Why lower OFC metabolism should predict treatment outcome is not clear. Lower OFC metabolism may, for example, be related to the recruitment of this region in behavioral control and inhibition.35,36 OFC metabolism may reflect the degree of control that patients feel they must exert on their behavior in order to satisfy their compulsions. It also is not evident why OFC metabolism may predict opposite results for psychotherapy and pharmacotherapy. Finally, because none of the studies predicting outcome in depression contrast psychotherapy and pharmacotherapy, it is not known whether activity in the rostral ACC in depression may differentially predict outcome depending on the treatment type as the OFC may in OCD.
Role of Awareness: Implications of Conscious and Unconscious Processing for Psychopathology and Psychotherapy
Most theories of psychopathology, both psychodynamic and cognitive, emphasize the importance of unconscious mental processes.37–39 This is particularly manifest in anxiety disorders. For all of their differences, these theories generally agree that anxiety-relevant unconscious processes 1) should differ between individuals with different anxiety levels; and 2) are probably the sum of a family of processes occurring outside of subjective awareness. A recent fMRI study40 has investigated these features of unconscious processing in anxiety. The results shed light on the conscious and unconscious components of anxiety and delineate new ways of evaluating the mechanisms whereby anxious subjects respond to treatment. Etkin et al.40 examined a group of normal volunteers and exposed them to fearful faces. While none of these participants had anxiety disorders, they represented the range of baseline (trait) anxiety present in the normal population. Fearful facial expressions are culturally conserved signals of threat that have been shown to stimulate activity in the amygdala.41,42 These faces were presented for conscious or unconscious processing; the latter was achieved through backward masking, which entails presenting a fearful face very rapidly and is immediately followed by the presentation of a neutral face. This procedure renders stimuli consciously nonreportable. While viewing these faces, subjects were engaged in a color identification task not related to the emotion expressed on the face. Reaction times in this task could show the effects of emotion, if processing the emotional content affected the attentional resources available for the color identification task.
Etkin et al.40 found that individual differences in trait anxiety predicted activity in the amygdala, as well as reaction times and activity in cortical regions important in attention (Figure 4 and Figure 5). However, the relationships of brain activity or behavior to trait anxiety were seen only when stimuli were processed unconsciously. Etkin et al. therefore identified a network of brain regions important in the unconscious emotional vigilance commonly described behaviorally for nonclinically anxious individuals and patients with anxiety disorder (Figure 5).43 This network involved the amygdala in emotional evaluation and the dorsolateral prefrontal cortex and the posterior cingulate cortex in directing attention for better processing of the unconscious threat. The network also contained a number of ventral visual regions important in the processing of objects, particularly faces. Enhanced activity in these visual regions is thought to reflect the effects of enhanced attention. Emotional arousal led to faster color identification reaction times, but, again, only when emotion was processed unconsciously.
The fact that brain activations and reaction times were not correlated with anxiety during consciously processed fear illustrates another important point: conscious processes may secondarily regulate unconscious biases. As the subjects studied by Etkin et al. were free of clinical anxiety disorders, the failure of proper behavioral control by conscious processes may underlie some clinical anxiety disorders, which would allow unconscious biases to affect thoughts and behavior to a maladaptive extent. One such disorder is posttraumatic stress disorder (PTSD). In response to symptom provocation, patients with PTSD, compared to trauma-exposed non-PTSD individuals, commonly overactivate the amygdala and underactivate the medial prefrontal cortex, including the rostral ACC.44 Amygdala hyperactivation also occurs in response to unconsciously processed stimuli.45 More recently, Shin et al.46 presented patients with PTSD images of fearful and happy faces from the same image set used by Etkin et al.40 and found that fearful faces led to greater amygdala activation and hypoactivation of the ACC in patients, when matched against comparison subjects. The degree of hypoactivation of the rostral ACC was predicted by patients’ symptoms, suggesting that rostral ACC hypofunction during the conscious processing of generic threat stimuli may be one reason why patients with PTSD cannot consciously regulate their unconscious anxiety-related biases. As discussed earlier, one way in which the rostral ACC may regulate behavior is through the recruitment of cognitive control under conditions of emotional conflict.
Distinguishing between conscious and unconscious processes may be essential for understanding both psychopathology and psychotherapy. For example, certain therapies (or medications) may alter the capacity for secondary regulation of unconscious biases (e.g. activity in the rostral ACC or connected areas), but not the biases per se. Alternatively, the effectiveness of a therapy may relate to its ability to normalize unconscious biases. One can imagine two simple processes by which biases may be corrected. Excessive unconscious amygdala activation brought about by anxiety may be normalized through changes occurring primarily in the amygdala or by recruitment of additional areas, perhaps top-down inhibitory areas in the frontal cortex. Thus, neuroimaging can help distinguish conscious from unconscious brain changes and identify which particular brain changes are responsible for the behavioral improvement.
Early Development, Psychotherapy, and the Promise of Prevention
One of psychiatry’s ultimate goals is the prevention of mental illness. This will require new insights into the natural history of the disease, the premorbid vulnerabilities and prodromes that identify individuals at risk for a certain illness. Neuroimaging studies have begun to focus on three developmental factors that predispose to psychiatric disorders: 1) vulnerability genes for anxiety and mood disorders; 2) temperament, or innate emotional response biases; and 3) histories of abuse or trauma. Twenty years ago, Jerome Kagan identified infants with temperamental shyness and inhibition47 and found that this trait is heritable48 and relatively stable into young adulthood.49 Inhibited babies, who can be identified during the first year of life by measures of behavioral inhibition and autonomic and hypothalamic-pituitary-adrenal cortical hyper-reactivity to novelty, are at risk of developing social anxiety disorder (social phobia) as children and adolescents and generalized anxiety disorder as adults. The opposite temperament (uninhibited) refers to children who readily approach new people and situations. This early trait has its developmental downside as well: adolescents who were uninhibited as young children are at risk of impulsive, aggressive, and antisocial behaviors, and possibly bipolar disorder.50
To understand the neurobiological differences between these opposite vulnerability traits, Schwartz et al49 used fMRI to study young adults who had been identified as inhibited or uninhibited during the second year of life. They probed subjects’ reactions to novelty by showing these young people novel and familiar human faces. The subjects who were inhibited as toddlers responded with significantly greater activation of both amygdalae to novel relative to familiar faces, compared to those who were uninhibited as toddlers. Indeed, the subjects who were uninhibited children showed no significant difference at all in response to novel versus familiar faces. These findings demonstrate that certain behavioral traits, which can be identified in infancy and carry a risk of psychopathology, are characterized by persistent differences in specific brain circuits involved in processing emotional responses to novelty and uncertainty. Behaviorally inhibited children have also been found to have lower centroparietal cortical activation in response to angry or neutral faces.51
One of the most common and disabling psychiatric sequelae of inhibited temperament is social anxiety disorder52 Social anxiety disorder, also called social phobia, is the third most common psychiatric disorder,53 with an estimated lifetime prevalence of 3% to 13%.54 Children with high social anxiety misclassify angry facial expressions,55 and adults with social phobia56 show a heightened amygdala response to angry faces. Taken together, these findings suggest that shyness and behavioral inhibition in children are a developmentally early phenotype of a set of vulnerability genes for social anxiety disorder. The neurophysiologic mechanisms of this phenotype appear to involve hypoactivation of cortical areas important for processing negatively valenced facial expressions and hyperactivation of the amygdala. Thus, there may be an excess of bottom-up, as opposed to top-down, responding to threatening or distressing cues in these conditions. The interaction between cortical and subcortical systems may determine the pathology’s precise form and whether it is amenable to treatment.57
The adverse effects of behavioral inhibition can be moderated by an early rearing environment. For example, although inhibited 18-month olds have greater cortisol responses to the “Strange Situation,” a laboratory brief separation paradigm, than do uninhibited controls, a secure mother/infant attachment lowers inhibited infants’ cortisol responses to a level closer to that of the noninhibited infants.58 In addition, the stability of the behavioral inhibition trait over time also has been linked to parenting style. Specifically, behavioral inhibition in toddlerhood predicts reticence with unfamiliar peers at four years of age only if the toddlers’ mothers were intrusive and made derisive comments. If mothers were neither intrusive nor derisive then their children’s inhibition in toddlerhood was not significantly associated with peer reticence at four years.59
These data have implications for therapy and even prevention. Pharmacotherapy and psychotherapy are both effective in the treatment of social anxiety disorder. Effective pharmacotherapies include paroxetine and venlafaxine, which inhibit the reuptake of monoamines by presynaptic neurons.60 Among psychotherapies, cognitive-behavioral therapy has proven useful for adults.61 Modifications of cognitive-behavioral therapy specifically intended to prevent the chronic morbidity of social anxiety disorder in children and adolescents, such as Skills for Social and Academic Success (SASS), a school-based intervention, are being developed.62
The success of cognitive-behavioral therapy in both adults and children may relate to its ability to help anxious people disengage their attention from threatening stimuli.63,64 Thus, cognitive psychotherapies for social anxiety may facilitate top-down control (i.e., the ability to shift appropriately attention in the face of perceived social threats, an ability that inhibited children and socially phobic adults lack). Indeed, the structured learning that occurs in cognitive-behavioral therapy or SASS may supplement the learning that normally occurs in the context of a secure attachment to one’s mother or an adaptive parenting style. The success of pharmacotherapy may rely on different mechanisms, as discussed earlier. Thus, it may be possible to tailor the choice of an appropriate treatment modality to individual patients based on neuroimaging-guided assessment of their reaction to novelty and capacity for cognitive control. Using neuroimaging in this way would therefore help prevent particular disorders, rather than treat them once they have developed.
CONCLUSION
There is no longer any doubt that psychotherapy can result in detectable changes in the brain. Although the biological study of psychotherapy has barely begun, several lines of evidence point to an important role for neuroimaging in evaluating the mechanisms of disease pathogenesis, in following the success of therapeutic interventions, and in the pretherapeutic prediction of outcome. We propose several points that may guide future work in this area.
First, the postpsychotherapy changes in brain function detectable with neuroimaging must now be more systematically explored. For example, the results described above suggest important mechanistic differences between processes operating at conscious and unconscious levels. Such distinctions must be made in considering the mechanisms of action of psychotherapy and the cognitive resources individuals possess coming into their therapy.
Second, much clinical utility may be gained from the identification of pretherapeutic brain marker predictors of treatment success. The pretreatment activity of the anterior cingulate and orbitofrontal cortices have been found to predict the degree that a patient will improve after the pharmacological treatment of depression and OCD, respectively. These two regions may also be prognostic for the outcome of psychotherapy, as they are involved in conflict detection and resolution, and in behavioral inhibition—functions that may be key targets of psychotherapy. It will be important, therefore, to determine which brain regions are predictive of outcome in each disease, whether prognostic regions are specific to a disease or to a form of treatment, and whether a differential response to medication versus psychotherapy can be predicted before treatment. In addition, understanding the brain functions that best predict treatment response may allow the development of pharmacological agents that specifically enhance activity within prognostic regions. Combining novel pharmacological agents with psychotherapy will clearly be beneficial in leading to improved outcome.1
Finally, new biological insights into the predispositions to psychopathology, such as by early-life behavioral inhibition and shyness, may provide opportunities for preventive psychotherapeutic or pharmacological interventions, which might arrest pathogenic processes early in their development. Further investigation of the biology of these predispositions may thus aid in the development of novel therapies. We now need to focus on the best neuroimaging approaches in three areas: to guide individualized therapeutic choices, to track patients’ progress, and to use this information to predict recovery and likelihood for relapse.
ACKNOWLEDGMENTS
Some of the material in this article is also described in a chapter by three of the authors (A.E., C.P., and E.R.K.) and will appear in the American Psychiatric Publishing Textbook of Personality Disorders, May 2005.
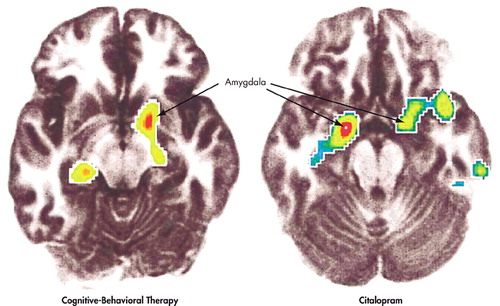
FIGURE 1. Effects of Cognitive-Behavioral Therapy (CBT) or Citalopram Treatment on Brain Activity in Patients With Social Phobia While Carrying Out a Public Speaking Task. Cognitive-Behavioral Therapy (left) and Citalopram (right) Treatment are Both Associated With Decreased Activation of the Amygdala During Performance of an Anxiogenic Public Speaking Task After Therapy, Compared With Before Therapy. Depicted Are Regions Showing a Significant Postversus Pretreatment Decrease in Activity. (Reprinted with permission from Furmark et al. 2002.)
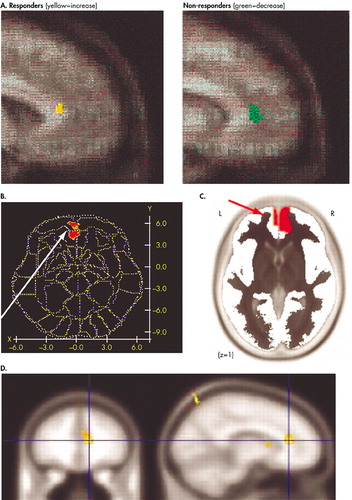
FIGURE 2. Prediction of Better Outcome in Depression by Higher Pretreatment Levels of Rostral Anterior Cingulate Cortex Metabolism or Activity. A) Responders to Antidepressants Were Found to be Hypermetabolic in the Rostral Cingulate, Whereas Nonresponders Were Hypometabolic (20). Basal Brain Metabolism (23) and Theta Frequency Electroencephalogram (EEG) Signal (24) in the Rostral Cingulate Predicted Better Outcome to Sleep Deprivation Therapy B) or Antidepressants C), Respectively. D) Greater Functional Recruitment of the Rostral Cingulate in an Emotional Activation Task Prior to Antidepressant Treatment Also Predicted Better Outcome (25). (Reprinted with permission from sources cited.)
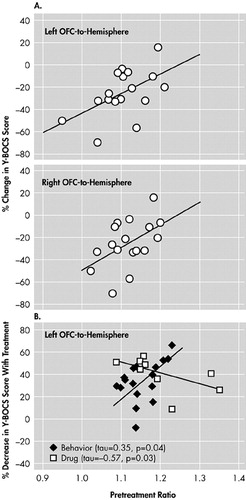
FIGURE 3. Prediction of Outcome of Pharmacotherapy or Psychotherapy for Obsessive-Compulsive Disorder by Pretreatment Orbitofrontal Cortex (OFC) Metabolism. A) Lower Pretreatment Metabolism in the OFC Predicted Better Response to Antidepressants (34), Reflected by a Greater Decrease in Yale-Brown Obsessive-Compulsive ScaleY-BOCS Scores, a Measure of OCD Symptoms. B) The Relationship Between Pretreatment OFC Metabolism and Outcome of Behavioral Psychotherapy (7) May be Opposite That Between Pretreatment OFC Metabolism and Outcome of Drug Therapy. (Reprinted with permission from sources cited.)
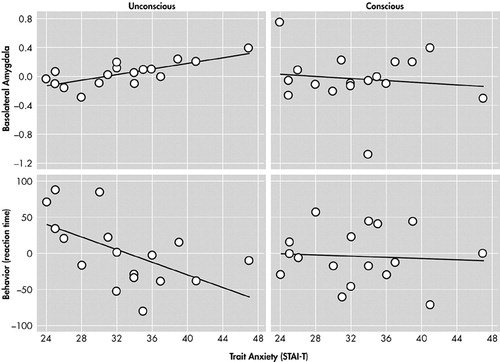
FIGURE 4. Prediction of Basolateral Subregion Amygdalar Activity by Differences in Baseline (trait) Anxiety Within a Normal Population During Unconscious, but Never Conscious, Processing of Fearful Faces. Individual Differences in Baseline (trait) Anxiety Predicted Activity in the Basolateral Subregion of the Amygdala During Unconscious, but not Conscious, Processing of Fearful Faces. Similarly, Differences in Trait Anxiety Predicted Behavior (color identification reaction times) Specifically During Unconscious Processing. (STAI-T=Spielberger State-Trait Anxiety Inventory—Trait Anxiety.)
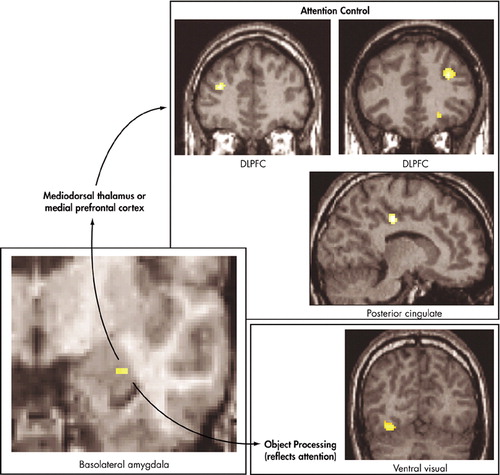
FIGURE 5. Identification of an Unconscious Emotional Vigilance Network in Anxiety.40 Unconscious Trait Anxiety–Correlated Activations are Seen in the Basolateral Amygdala, Dorsolateral Prefrontal Cortex (DLPFC), Posterior Cingulate, and a Number of Ventral Visual Areas. These Regions May Thus Form an Unconscious Emotional Vigilance Network That Directs Attention for Enhanced Processing of Unconscious Threat (DLPFC and posterior cingulate), Which Was Reflected in Enhanced Activation of Object Processing Areas in the Ventral Visual Atream.
1 Ressler KJ, Rothbaum BO, Tannenbaum L, et al: Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004; 61(11):1136–1144Crossref, Medline, Google Scholar
2 Elkin I, Shea MT, Watkins JT, et al.: National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry 1989; 46(11):971–982Crossref, Medline, Google Scholar
3 March J, Silva S, Petrycki S, et al: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. Jama 2004; 292(7):807–820Crossref, Medline, Google Scholar
4 Rector NA: Cognitive behavioural therapy reduces short term rehospitalisation compared with psychoeducation in inpatients with schizophrenia. Evid Based Ment Health 2005; 8(1):8Crossref, Medline, Google Scholar
5 Kandel ER: Psychotherapy and the single synapse. The impact of psychiatric thought on neurobiologic research. N Engl J Med 1979; 301(19):1028–1037Crossref, Medline, Google Scholar
6 Baxter LR, Jr., Schwartz JM, Bergman KS, et al.: Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49(9):681–689Crossref, Medline, Google Scholar
7 Brody AL, Saxena S, Schwartz JM, et al: FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res 1998; 84(1):1–6Crossref, Medline, Google Scholar
8 Brody AL, Saxena S, Stoessel P, et al: Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry 2001; 58(7):631–640Crossref, Medline, Google Scholar
9 Martin SD, Martin E, Rai SS, et al: Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry 2001; 58(7):641–648Crossref, Medline, Google Scholar
10 Schwartz JM, Stoessel PW, Baxter LR, Jr., et al: Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53(2):109–113Crossref, Medline, Google Scholar
11 Drevets WC: Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med 1998; 49:341–361Crossref, Medline, Google Scholar
12 Kennedy SH, Javanmard M, Vaccarino FJ: A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry 1997; 42(5):467–475Crossref, Medline, Google Scholar
13 Mayberg HS: Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997; 9(3):471–481Link, Google Scholar
14 Mayberg HS, Liotti M, Brannan SK, et al: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156(5):675–682Medline, Google Scholar
15 Goldapple K, Segal Z, Garson C, et al: Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004; 61(1):34–41Crossref, Medline, Google Scholar
16 Furmark T, Tillfors M, Marteinsdottir I, et al: Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 2002; 59(5):425–433Crossref, Medline, Google Scholar
17 Paquette V, Levesque J, Mensour B, et al: "Change the mind and you change the brain": effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage 2003; 18(2):401–409Crossref, Medline, Google Scholar
18 Tillfors M, Furmark T, Marteinsdottir I, et al: Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001; 158(8):1220–1226Crossref, Medline, Google Scholar
19 Meyer-Lindenberg A, Poline JB, Kohn PD, et al: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158(11):1809–1817Crossref, Medline, Google Scholar
20 Mayberg HS, Brannan SK, Mahurin RK, et al: Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8(4):1057–1061Crossref, Medline, Google Scholar
21 Saxena S, Brody AL, Ho ML, et al: Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry 2003; 160(3):522–532Crossref, Medline, Google Scholar
22 Volk SA, Kaendler SH, Hertel A, et al: Can response to partial sleep deprivation in depressed patients be predicted by regional changes of cerebral blood flow? Psychiatry Res 1997; 75(2):67–74Crossref, Medline, Google Scholar
23 Wu J, Buchsbaum MS, Gillin JC, et al: Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 1999; 156(8):1149–1158Medline, Google Scholar
24 Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al: Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 2001; 158(3):405–415Crossref, Medline, Google Scholar
25 Davidson RJ, Irwin W, Anderle MJ, et al: The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160(1):64–75Crossref, Medline, Google Scholar
26 Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4(6):215–222Crossref, Medline, Google Scholar
27 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118 (Pt 1):279–306Google Scholar
28 Davidson RJ, Pizzagalli D, Nitschke JB, et al: Depression: perspectives from affective neuroscience. Annu Rev Psychol 2002; 53:545–574Crossref, Medline, Google Scholar
29 Drevets WC: Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 2001; 11(2):240–249Crossref, Medline, Google Scholar
30 Whalen PJ, Bush G, McNally RJ, et al: The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 1998; 44(12):1219–1228Crossref, Medline, Google Scholar
31 Kerns JG, Cohen JD, MacDonald AW, 3rd, et al: Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303(5660):1023–6Crossref, Medline, Google Scholar
32 McGuire PK, Bench CJ, Frith CD, et al: Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry 1994; 164(4):459–68Crossref, Medline, Google Scholar
33 Rauch SL, Jenike MA, Alpert NM, et al: Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994; 51(1):62–70Crossref, Medline, Google Scholar
34 Saxena S, Brody AL, Maidment KM, et al.: Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 1999; 21(6):683–93Crossref, Medline, Google Scholar
35 Horn NR, Dolan M, Elliott R, et l: Response inhibition and impulsivity: an fMRI study. Neuropsychologia 2003; 41(14):1959–66Crossref, Medline, Google Scholar
36 Lubman DI, Yucel M, Pantelis C: Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction 2004; 99(12):1491–502Crossref, Medline, Google Scholar
37 Beck AT, Clark DA: An information processing model of anxiety: automatic and strategic processes. Behav Res Ther 1997; 35(1):49–58Crossref, Medline, Google Scholar
38 Gabbard GO: Basic principles of dynamic psychiatry. Washington, DC, American Psychiatric Press, Inc., 2000Google Scholar
39 Wong PS: Anxiety, signal anxiety, and unconscious anticipation: neuroscientific evidence for an unconscious signal function in humans. J Am Psychoanal Assoc 1999; 47(3):817–41Crossref, Medline, Google Scholar
40 Etkin A, Klemenhagen KC, Dudman JT, et al: Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron 2004; 44(6):1043–55Crossref, Medline, Google Scholar
41 Aggleton JP: The amygdala: a functional analysis. New York (USA), Oxford University Press, 2000Google Scholar
42 Ekman P, Sorenson ER, Friesen WV: Pan-cultural elements in facial displays of emotion. Science 1969; 164(875):86–88Crossref, Medline, Google Scholar
43 Mogg K, Bradley BP: A cognitive-motivational analysis of anxiety. Behav Res Ther 1998; 36(9):809–48Crossref, Medline, Google Scholar
44 Hull AM: Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry 2002; 181:102–110Crossref, Medline, Google Scholar
45 Rauch SL, Whalen PJ, Shin LM, et al: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47(9):769–776Crossref, Medline, Google Scholar
46 Shin LM, Wright CI, Cannistraro PA, et al: A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005; 62(3):273–281Crossref, Medline, Google Scholar
47 Kagan J, Reznick JS, Snidman N: The physiology and psychology of behavioral inhibition in children. Child Dev 1987; 58(6):1459–1473Crossref, Medline, Google Scholar
48 Emde RN, Plomin R, Robinson JA, et al: Temperament, emotion, and cognition at fourteen months: the MacArthur Longitudinal Twin Study. Child Dev 1992; 63(6):1437–1455Crossref, Medline, Google Scholar
49 Schwartz CE, Wright CI, Shin LM, et al: Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science 2003; 300(5627):1952–1953Crossref, Medline, Google Scholar
50 Hirshfeld-Becker DR, Biederman J, Calltharp S, et al: Behavioral inhibition and disinhibition as hypothesized precursors to psychopathology: implications for pediatric bipolar disorder. Biol Psychiatry 2003; 53(11):985–99Crossref, Medline, Google Scholar
51 Battaglia M, Ogliari A, Zanoni A, et al: Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Arch Gen Psychiatry 2005; 62(1):85–94Crossref, Medline, Google Scholar
52 Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al: Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry 2001; 158(10):1673–1679Crossref, Medline, Google Scholar
53 Pollack MH: Comorbidity, neurobiology, and pharmacotherapy of social anxiety disorder. J Clin Psychiatry 2001; 62 Suppl 12:24–29Google Scholar
54 APA: Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC, American Psychiatric Press, Inc., 2000Google Scholar
55 Battaglia M, Ogliari A, Zanoni A, et al: Children's discrimination of expressions of emotions: relationship with indices of social anxiety and shyness. J Am Acad Child Adolesc Psychiatry 2004; 43(3):358–365Crossref, Medline, Google Scholar
56 Stein MB, Goldin PR, Sareen J, et al: Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59(11):1027–1034Crossref, Medline, Google Scholar
57 Fox NA, Henderson HA, Marshall PJ, et al: Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol 2005; 56:235–262Crossref, Medline, Google Scholar
58 Nachmias M, Gunnar M, Mangelsdorf S, et al: Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev 1996; 67(2):508–522Crossref, Medline, Google Scholar
59 Rubin KH, Burgess KB, Hastings PD: Stability and social-behavioral consequences of toddlers' inhibited temperament and parenting behaviors. Child Dev 2002; 73(2):483–495Crossref, Medline, Google Scholar
60 Liebowitz MR, Gelenberg AJ, Munjack D: Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry 2005; 62(2):190–198Crossref, Medline, Google Scholar
61 Westenberg HG, Liebowitz MR: Overview of panic and social anxiety disorders. J Clin Psychiatry 2004; 65 Suppl 14:22–26Google Scholar
62 Fisher PH, Masia-Warner C, Klein RG: Skills for social and academic success: a school-based intervention for social anxiety disorder in adolescents. Clin Child Fam Psychol Rev 2004; 7(4):241–249Crossref, Medline, Google Scholar
63 Fox E, Russo R, Dutton K: Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cogn emot 2002; 16(3):355–379Crossref, Medline, Google Scholar
64 Derryberry D, Reed MA: Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol 2002; 111(2):225–236Crossref, Medline, Google Scholar



