Neuropsychological Functioning in Early- and Late-Onset Obsessive-Compulsive Disorder
Abstract
Significant relationships have been noted between age of onset and demographics, clinical characteristics, and cerebral metabolic activity in obsessive-compulsive disorder (OCD). The authors investigated whether patients with early (N=21) and late (N=17) onset OCD differ with respect to neuropsychological functioning. Results revealed that the late onset OCD group obtained poorer scores on measures of executive function and auditory attention than the early onset group. Late onset OCD was also associated with poorer visual memory relative to healthy comparison subjects. These findings suggest that early and late onset OCD may be the result of at least partially differing neurobiological mechanisms.
Childhood or adolescent onset is reported by approximately one third to one-half of adults with obsessive-compulsive disorder (OCD).1 Presently, it is unclear whether early and late onset OCD are different subtypes of the disorder or are part of a developmental continuum. Differences related to age of onset have been observed for sex distribution, family history of psychiatric illness, symptom subtypes, treatment response, and prevalence of comorbid psychiatric disorders.2–5
Converging lines of evidence have implicated abnormality of frontostriatal circuitry in the etiology of OCD. Functional neuroimaging studies have generally reported hyperactivation of the orbitofrontal cortex, cingulate gyrus, and caudate nucleus.6 Structural neuroimaging studies have been less consistent, with some investigations reporting abnormal volumes of the caudate nucleus and orbitofrontal cortex relative to healthy comparison subjects.7–9 Neuropsychological studies have shown abnormalities in several cognitive domains in children and adults with OCD, including executive functions, memory, and visuospatial skills, albeit inconsistently.10–12
The relationship between age of onset and structural and functional brain integrity has received scant empirical investigation. Patients with early and late onset OCD have been reported to show different patterns of motor control abnormalities during handwriting,13 but did not differ on tests of verbal and visual memory.14 In a single photon emission tomography (SPECT) study, patients with early onset OCD showed abnormal resting blood flow in the anterior cingulate gyrus, orbitofrontal cortex and cerebellum, while patients with late onset showed abnormalities in orbitofrontal cortex and precuneus, relative to healthy comparison subjects.15 Another SPECT study failed to find any correlation between age of onset and resting blood flow.16
In the present study, we extended prior research by evaluating patients with early and late onset OCD on several domains of neuropsychological functioning. Late onset has been defined from 13 years of age15 to 18 years of age14 and older. We chose to define late onset OCD as having onset of symptoms at 13 years of age or older, consistent with a SPECT study reporting significant differences between early and late onset OCD using this cutoff,15 as well as to separate childhood onset from that occurring in adolescence or adulthood. Potential interactions between sex and age of onset on neuropsychological functioning were also evaluated, given prior evidence of a differential relationship between sex and age of onset in the disorder.2,17
METHOD
Participants
Participants were 21 outpatients with OCD reporting onset of the disorder at 12 years of age or under (early onset group), 17 outpatients with OCD with reported onset at age 13 years or older (late onset group), and 27 healthy comparison subjects. Patients and comparison subjects were between 18 and 65 years of age, Caucasian, and had no history of neurological illness, substance abuse, head injury resulting in a loss of consciousness, ECT or psychosurgery, or systemic illness with potential effects on cognitive functioning. Patients were evaluated by both a psychiatrist and a psychologist with extensive experience in diagnosing OCD, using the Anxiety Disorders Interview Schedule.18 Patients were excluded from the present investigation if they had a comorbid psychiatric disorder other than a secondary anxiety or depressive disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition.19 Patients were also administered the Yale-Brown Obsessive Compulsive Scale (YBOCS), a structured interview specifically designed to determine the nature, extent and severity of obsessions and compulsions.20 Six participants in each of the patient groups were receiving a selective serotonin reuptake inhibitor at the time of participation. The remaining patients were unmedicated. None of the patients were participating in psychotherapy at the time of participation. Healthy comparison subjects were recruited from a subject pool at the Centre de Recherche Fernand Séguin (a specialized research center affiliated with the University of Montreal, Quebec, Canada, and Louis H. Lafontaine Hospital) and advertisements in a local newspaper. Comparison subjects had no personal history of psychiatric illness per self-report. After complete description of the study to subjects, written informed consent was obtained.
Procedure
Neuropsychological tests and self-report mood measures were administered in a fixed order in a single session of approximately three and one-half hours. Mood measures included the Beck Depression Inventory21 and the State-Trait Anxiety Inventory-State Scale.22 Neuropsychological domains evaluated included executive functions (percent perseverative errors on the Wisconsin Card Sorting Task [WCST];23 total number of errors on the 12-item abstract designs version of the Self-Ordered Pointing Task;24 time to complete trial B of the Trail Making Test (TMT-B);25 verbal memory (mean number of items recalled on immediate recall Logical Memory [LM]-I and following a 30-minute delay [LM-II] on the LM subtest of the Wechsler Memory Scale—Revised [WMS-R];26 total number of words recalled over the five learning trials and the 30-minute delayed recall trial of the Rey Auditory-Verbal Learning Test (RAVLT);27 visual memory (WMS-R Visual Reproduction immediate [VR-I] and 30-minute [VR-II] delayed recall score);26 Rey Complex Figure 30-minute delayed recall, total score out of 36;28 visuoconstruction (total score on the Block Design subtest of the WAIS–Revised [WAIS-R]);29 copy of the Rey Complex Figure, total score out of 36;28 psychomotor speed/attention (Digit Span and Digit Symbol subtest age-scaled scores from the WAIS–Revised;29 time to complete part A of the Trail Making Test (TMT-A);25 and motor skills (mean left and right handgrip strength in kilograms on the hand dynamometer;25 mean left and right hand motor speed on the Purdue Pegboard Test.30
Data Analysis
Group differences in demographic characteristics were analyzed using independent samples t tests, analysis of variance (ANOVA), and nonparametric statistics as appropriate. Analysis of variance was conducted to evaluate group differences on the individual neuropsychological test scores. The presence of sex effects on neuropsychological measures was analyzed using a two-way group-by-sex ANOVA. Significant ANOVAs were followed by post hoc analyses using Tukey’s least significant difference (LSD) test. This permitted identification of specific group differences while controlling for potential type I error. All tests used two-tailed comparisons with significance level set at p<0.05.
RESULTS
Demographic and clinical characteristics of the patient and comparison groups are presented in Table 1. Group differences for age (F=2.65, df=2, 62, p=0.08), sex composition (χ2=2.16, df=2, p=0.34) and handedness (χ2=1.28, df=2, p=0.53) were not significant. However, the late onset group completed significantly fewer years of education than the comparison subjects (F=4.30, df=2, 62, p=0.02). The patient groups were also significantly more depressed (F=29.91, df=2, 62, p=0.001) and anxious (F=12.32, df=2,62, p=0.001) than the comparison subjects. The early and late onset patient groups did not differ with respect to symptom severity (F=0.41, df=1, 36, p=0.97) on the YBOCS, percentage of patients with comorbid depressive (χ2=1.59, df=1, p=0.26) or anxiety disorders (χ2=2.34, df=1, p=0.18), or percentage of patients receiving psychotropic medication (χ2=0.49, df=1, p=0.49).
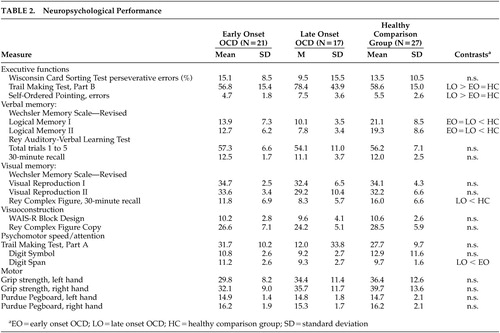
Descriptive statistics for neuropsychological test variables are presented in Table 2. Results revealed that both patient groups recalled significantly less information on LM-I (F=14.09, df=2,62, p=0.001) and LM-II (F=15.53, df=2,62, p=0.001) than the comparison group. The late onset group also took longer to complete TMT-B (F=4.00, df=2,62, p=0.02) and made more errors on the Self-Ordered Pointing Test (F=5.33, df=2,62, p=0.007) than both the early onset and comparison groups, showed poorer delayed recall of the Rey Complex Figure (F=7.63, df=2,62, p=0.001), and had a lower digit span (F=4.04, df=2,62, p=0.02) than the early onset group. All group differences remained significant when controlling for education, depression or anxiety using analysis of covariance (p<0.05). No significant group × sex interaction was observed for any of the neuropsychological variables (p>0.05).
DISCUSSION
Results of the present study indicate that patients with early and OCD differ in their pattern of neuropsychological functioning. Patients with early onset OCD only showed poorer memory for prose passages relative to healthy comparison subjects. In contrast, patients with late onset OCD showed poorer performance on tests of verbal and visual memory, as well as executive functions and auditory attention. These findings support results of a recent SPECT study indicating differences in cerebral blood flow related to age of onset in OCD.15
The observation of impaired executive functions in late onset OCD is consistent with a hypothesized “primary” subtype of the disorder that is characterized by late onset and frontal systems dysfunction.2 Executive dysfunction has been observed in several studies of OCD patients that were not classified based on age of onset.12,31 However, a number of recent studies have found that neuropsychological differences between patients with OCD and healthy comparison subjects, particularly executive dysfunction, are accounted for by depression.32,33 In the present study, the patient groups did not differ with respect to self-reported mood or comorbid depressive or anxiety disorders. Furthermore, neuropsychological differences between the patient and comparison groups could not be accounted for by self-reported depression or anxiety. Thus, executive dysfunction in late onset OCD cannot be accounted for by co-occurring mood symptoms.
A neurodevelopmental subtype of OCD has also been proposed that is characterized by childhood onset, predominantly male sex, poorer response to treatment, and neuromotor abnormalities.34 While our sample sizes were modest, results indicate that sex does not moderate the relationship between age of onset and neuropsychological functioning. The lack of group differences for motor skills appears inconsistent with evidence of greater prevalence of tic disorders in early onset OCD35 and results of a recent study of handwriting in adults with OCD that found earlier onset to be associated with disturbed movement sequencing, while later age of onset is related to greater difficulty performing movements requiring more complex motor control.13 However, we employed relatively simple tasks involving handgrip strength and motor speed, neither of which required sequencing or complex movements. Further studies of early and late onset OCD employing motor tasks with sequencing and increased movement complexity requirements would likely prove informative. In addition, information regarding the presence of tic disorder in our sample was unavailable. Assessment of motor control in relation to age of onset and presence of tic disorder would be helpful to further evaluate the validity of a neurodevelopmental subtype of OCD.
Discrepancies between studies investigating correlates of age of onset in OCD may be due in part to the use of different cutoffs to define early and late onset groups. We defined late onset as that occurring at age 13 or later, based on results of a recent SPECT study15 and in order to separate childhood onset from that in adolescence or adulthood. While we believe that this cutoff is reasonable, based on the above arguments, we acknowledge that both our cutoff and that used by other authors is somewhat arbitrary. Studies with large samples of patients with OCD would permit the use of multivariate statistical techniques to identify significant peaks in onset of symptoms across the life span, thus allowing for a more empirically based classification. Furthermore, while a number of studies of adult patients have employed retrospective self-report of OCD symptom onset,14,36 our study must be interpreted in the context of the limitations inherent in this methodology.
We did not observe any difference in memory functioning between patients with early and late onset OCD, consistent with the only other published study to evaluate the relationship between age of onset and neuropsychological functioning.14 However, our late onset group showed poorer performance on a visual memory test, and both patient groups performed worse on a verbal memory test, relative to the comparison group. It is unknown whether this visual memory impairment in our late onset group would also have been observed by Henin et al. if they had included a healthy comparison group,14 or if they had used our cutoff of 12 years of age and younger rather their use of under 18 years of age for defining early onset OCD.
In summary, the present findings of neuropsychological differences between early and late onset OCD raise the possibility of differential neurobiological substrates related to age of onset. Discrepancies between prior neuropsychological studies of OCD may be due in part to inclusion of patients with widely varying ages of onset. Further studies examining neuropsychological functioning in early and late onset OCD in larger samples are warranted. The present findings support recent SPECT evidence of differential patterns of brain activation in early and late onset OCD.15 However, the precise neural circuitry underlying the patterns of cognitive functioning observed in the present investigation remains unclear and does not readily map onto the observed regional differences in the aforementioned SPECT study. Positron emission tomography and functional MRI during the performance of cognitive tasks would be helpful in directly evaluating the relationship between cognitive functions and underlying neural circuitry in early and late onset OCD.
ACKNOWLEDGMENTS
This study was supported by grants from the Fonds Pour La Formation de Chercheurs et L’Aide a la Recherche (RMR) and the Fond de la Recherche en Santé Québec (KO and JB).
 |
 |
1 Kolanda JL, Bland RC, Newman SC: Obsessive-compulsive disorder. Acta Psychiatr Scand Suppl 1994; 376:24–35Crossref, Medline, Google Scholar
2 Blanes T, McGuire P: Heterogeneity within obsessive-compulsive disorder: evidence for primary and neurodevelopmental subtypes, in Neurodevelopmental and Adult Psychopathology. Edited by Keshavan MS, Murray RM. New York, Cambridge University Press, 1997Google Scholar
3 Hollander E, DeCaria CM, Nitesai A, et al: Serotonergic function in obsessive-compulsive disorder: behavioral and neuroendocrine responses to oral m-chlorphenylpiperazine and fenfluramine in patients and normal volunteers. Arch Gen Psychiatry 1992; 49:21–28Crossref, Medline, Google Scholar
4 Minichiello WE, Baer L, Jenike MA, et al: Age of onset of major subtypes of obsessive-compulsive disorder. J Anxiety Disord 1990; 4:147–150Crossref, Google Scholar
5 Ravizza L, Barzega G, Bellino S, et al: Predictors of drug treatment response in obsessive-compulsive disorder. J Clin Psychiatry 1995; 56:368–373Medline, Google Scholar
6 Saxena S, Rauch SL: Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23:563–586Crossref, Medline, Google Scholar
7 Aylward EH, Harris GJ, Hoehn-Saric R, et al: Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Arch Gen Psychiatry 1996; 53:577–584Crossref, Medline, Google Scholar
8 Robinson D, Wu H, Munne RA, et al: Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry 1995; 52:393–398Crossref, Medline, Google Scholar
9 Rosenberg DR, Keshavan MS, O’Hearn KM et al: Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry 1997; 54:824–830Crossref, Medline, Google Scholar
10 Alarcon RD, Libb JW, Boll TJ: Neuropsychological testing in obsessive-compulsive disorder: a clinical review. J Neuropsychiatry Clin Neurosci 1994; 6:217–228Link, Google Scholar
11 Purcell R, Maruff P, Kyrios M, et al: Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry 1998; 43:348–357Crossref, Medline, Google Scholar
12 Savage CR, Baer L, Keuthen NJ, et al: Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry 1999; 45:905–916Crossref, Medline, Google Scholar
13 Mavrogiorgou P, Mergl R, Tigges P, et al: Kinematic analysis of handwriting movements in patients with obsessive-compulsive disorder. J Neurol Neurosurg Psychiatry 2001; 70:605–612Crossref, Medline, Google Scholar
14 Henin A, Savage CR, Rauch SL, et al: Is age at symptom onset associated with severity of memory impairment in adults with obsessive-compulsive disorder? Am J Psychiatry 2001; 158:137–139Crossref, Medline, Google Scholar
15 Busatto GF, Buchpiguel CA, Zamignani DR, et al: Regional cerebral blood flow abnormalities in early-onset obsessive-compulsive disorder: an exploratory SPECT study. J Am Acad Child Adolesc Psychiatry 2001; 40:347–354Crossref, Medline, Google Scholar
16 Lucey JV, Costa DC, Blanes T, et al: Regional cerebral blood flow in obsessive-compulsive disordered patients at rest. Differential correlates with obsessive-compulsive and anxious-avoidant dimensions. Br J Psychiatry 1995; 167:629–634Crossref, Medline, Google Scholar
17 Noshirvani HF, Kasvikis Y, Marks IM, et al: Gender-divergent aetiological factors in obsessive-compulsive disorder. Br J Psychiatry 1991; 158:260–263Crossref, Medline, Google Scholar
18 Brown TA, Di Nardo PA, Barlow DH: Anxiety Disorders Interview Schedule for DSM-IV. Albany, NY, Graywind Publications, 1994Google Scholar
19 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, APA, 1994Google Scholar
20 Goodman WK, Price LH, Rasmussen SA, et al: The Yale-Brown Obsessive-Compulsive Scale, I: development, use, and reliability. Arch General Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
21 Beck AT: Beck Depression Inventory. San Antonio, Tex, Psychological Corp, 1987Google Scholar
22 Spielberger C, Montouri E, Luschene R: State-Trait Anxiety Inventory (STAI Form C1). Palo Alto, Calif, Consulting Psychologists Press, 1970Google Scholar
23 Heaton RK, Chelune GJ, Talley JL, et al: Wisconsin Card Sorting Test (WCST) Manual Revised and Expanded. Odessa, Fla, Psychological Assessment Resources, 1993Google Scholar
24 Petrides M, Milner B: Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 1982; 20:249–262Crossref, Medline, Google Scholar
25 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
26 Wechsler D: Wechsler Memory Scale-Revised. San Antonio, Tex, Psychological Corp, 1987Google Scholar
27 Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
28 Rey A: L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychologie 1941; 28:286–340Google Scholar
29 Wechsler D: Wechsler Adult Intelligence Scale-Revised. Cleveland, Psychological Corp, 1981Google Scholar
30 Tiffin J, Asher EJ: The Purdue pegboard: norms and studies of reliability and validity. J Appl Psychol 1948; 32:234–247Crossref, Medline, Google Scholar
31 Purcell R, Maruff P, Kyrios M, et al: Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry 1998; 55:415–423Crossref, Medline, Google Scholar
32 Basso MR, Bornstein RA, Carona F, Morton R: Depression accounts for executive function deficits in obsessive-compulsive disorder. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:241–245Medline, Google Scholar
33 Moritz S, Birkner C, Kloss M, et al: Impact of comorbid depressive symptoms on neuropsychological performance in obsessive-compulsive disorder. J Abnorm Psychol 2001; 110:653–657Crossref, Medline, Google Scholar
34 Geller DA, Biederman J, Faraone S, et al: Developmental aspects of obsessive compulsive disorder: findings in children, adolescents, and adults. J Nerv Ment Dis 2001; 189:471–477Crossref, Medline, Google Scholar
35 Grados MA, Riddle MA, Samuels JF, et al: The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: the Hopkins OCD family study. Biol Psychiatry 2001; 50:559–565Crossref, Medline, Google Scholar
36 Sobin C, Blundell M, Weiller F, et al: Phenotypic characteristics of obsessive-compulsive disorder ascertained in adulthood. J Psychiatr Res 1999; 33:265–273Crossref, Medline, Google Scholar



