Dose Effects of Modafinil in Sustaining Wakefulness in Narcolepsy Patients With Residual Evening Sleepiness
Abstract
Excessive sleepiness associated with narcolepsy lasts throughout the waking day. The authors conducted two randomized, double-blind studies to compare the efficacy of modafinil once-daily versus split doses in maintaining wakefulness throughout the day. Fifty-six patients received modafinil. The split-dose regimens were significantly more effective than the 200-mg once-daily regimen for sustaining wakefulness in the late afternoon/evening. All modafinil dosing regimens were well tolerated. In patients who experience excessive sleepiness in the late afternoon/evening, despite satisfactory treatment earlier in the day, a split dose of modafinil may promote wakefulness throughout the waking day.
The wake-promoting agent modafinil is an effective and well-tolerated treatment for the improvement of wakefulness in patients experiencing excessive sleepiness associated with narcolepsy.1,2,3 Although the precise mechanism of action of modafinil has not been established, findings from preclinical studies suggest that modafinil may promote wakefulness through its selective action on areas of the brain responsible for maintaining normal wakefulness.4,5,6
In two 9-week, large-scale, double-blind, placebo-controlled clinical trials, once-daily administration of modafinil at doses of 200 mg and 400 mg has been shown to significantly enhance wakefulness through mid-afternoon.1,2 The relatively long elimination half-life of modafinil (12 to 14 hour)7 further supports a once-daily dosing regimen.
Despite these clinical and pharmacokinetic observations, some patients with narcolepsy who have satisfactory responses to modafinil in the early part of the day may experience sleepiness in the late afternoon or evening. Significant improvement in wakefulness has been demonstrated with split-dose regimens of modafinil in three previously conducted studies,3,8,9 but comparative efficacy versus once-daily regimens was not established. We recently completed two similar randomized, double-blind, parallel studies comparing the relative effects of various modafinil doses and dose regimens on wakefulness throughout the entire waking day in patients with narcolepsy experiencing residual late-afternoon/evening sleepiness following satisfactory responses earlier in the day. Here, we present a combined analysis of data from these two studies.
METHOD
Study Design
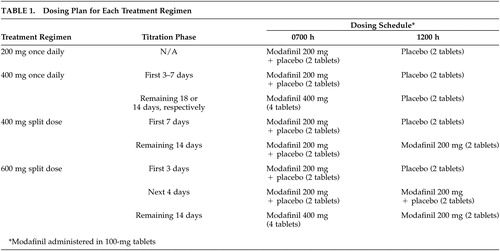
Data were pooled from two studies (one at one center, the other at three centers), using similar 3-week randomized, double-blind, parallel study designs. The institutional review boards at respective centers approved the protocol and all patients gave informed consent. Following a 1-week screening period, patients who met the inclusion criteria were randomized to 1 of 4 modafinil dose regimens for 3 weeks: 200 mg once daily (0700 hour), 400 mg once daily (0700 hour), 400 mg split dose (200 mg, 0700 hour; 200 mg, 1200 hour), and 600 mg split dose (400 mg, 0700 hour; 200 mg, 1200 hour). Morning doses (0700 hour) were given with breakfast, whereas midday doses (1200 hour) were given with lunch. For patients receiving 400 mg once daily, 400 mg split dose, and 600 mg split dose, modafinil was initiated at a dose of 200 mg and titrated upward (Table 1). Each study treatment period was preceded by a 1- or 2-week, single-blind, placebo washout period during which patients received matching placebo in the morning (0700 hour) and at noon. Patients visited their respective centers after completing the washout period (baseline) and at the end of 3 weeks of double-blind treatment.
Patients
Fifty-five patients aged 18 to 70 years and one patient aged 14 years with a current diagnosis of narcolepsy as defined by the American Sleep Disorders Association10 entered the study. All patients had historical nocturnal polysomnography and Multiple Sleep Latency Test (MSLT) data (i.e., within 5 years of screening), a positive therapeutic response to modafinil but residual late-afternoon or evening sleepiness, and a Clinical Global Impression of Severity (CGI-S)11 rating of ≥4 (at least moderately ill) with respect to late-afternoon/evening sleepiness at the screening visit. A more detailed description of patient selection criteria has been published previously.12 Most patients (N=46; 82%) were taking modafinil 400 mg/day; seven patients (13%), 200 mg/day; two patients (4%), 300 mg/day; and 1 patient (2%), 600 mg/day at screening.
ASSESSMENTS
Efficacy
Efficacy was assessed using an extended Maintenance of Wakefulness Test (MWT),13 the Clinical Global Impression of Change (CGI-C),11 and the Epworth Sleepiness Scale (ESS).14 The methodology associated with use of these assessments has been described previously.12 The MWT is an objective assessment of sleepiness that measures the ability of the patient to remain awake. The MWT has been modified various times in previous trials. In the current trials, individual test sessions were 30 minutes in duration and the testing period was extended throughout the day, including sessions conducted in the morning (0900 hour and 1100 hour), afternoon (1300 hour and 1500 hour), and evening (1700 hour and 1900 hour). The CGI can be used to assess the response to treatment by measuring the patient’s initial level of severity of clinical condition at baseline, or change relative to baseline, and helps physicians to understand the relevance of the clinical effects of increased wakefulness. The ESS is a validated patient questionnaire that measures the extent to which excessive sleepiness (ES) interferes with patients’ daily lives. For this assessment, the patient answers questions regarding the likelihood of falling asleep while performing eight common, nonstimulating activities. A brief physical examination and vital sign measurements were conducted after the 1500 hour session.
Safety
Adverse events and their severity (mild, moderate, or severe) and relationship to study medication were recorded throughout the study. Standard clinical laboratory tests, a complete physical examination, and a 12-lead electrocardiogram (ECG) were performed at screening and final visit.
Statistical Analysis
An analysis of covariance (ANCOVA) model using rank-transformed data,15 with treatment as a factor and baseline value as a covariate, was performed for comparisons between treatment groups for each continuous variable (i.e., mean change from baseline in the MWT sleep latency time for the six sessions from 0900 hour to 1900 hour; mean change from baseline in morning, afternoon, and evening sessions of the MWT; and mean change from baseline in ESS total score). Within-group comparisons between baseline and endpoint values were made using a paired t test on rank-transformed data. The proportion of patients remaining awake for the first 20 minutes of each MWT session was calculated, and between-group comparisons were performed using logistic regression analysis with treatment as a factor and baseline value as a covariate. Results of the CGI-C were analyzed using a proportional odds model with treatment as a factor and baseline value as a covariate. All statistical tests were two-sided and performed at the 5% level of significance. All patients who received the study drug were included in the safety analysis.
RESULTS
Patients
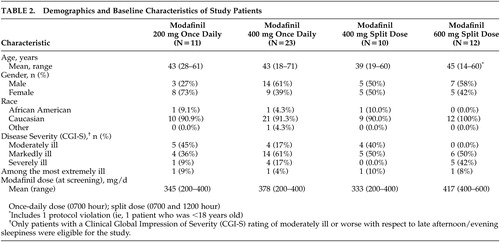
Of the 56 patients randomized to treatment, 11 received modafinil 200 mg once daily, 23 received modafinil 400 mg once daily, 10 received the modafinil 400-mg split-dose regimen, and 12 received the modafinil 600-mg split-dose regimen. The demographic and baseline characteristics of the patients in the four treatment groups were comparable (Table 2). Patients’ ages ranged from 14 to 71 years (mean ∼43 years). The majority (N=52; 93%) of the study patients were white, and slightly more than one-half were males (N=29; 52%). Most patients (77%; N=43) were markedly, severely, or extremely ill. All 56 patients completed the 3-week double-blind study period.
Efficacy Assessments
All modafinil doses and dosing regimens significantly improved patients’ ability to sustain wakefulness based on the total daily mean MWT sleep latency times at week 3 compared with baseline (p<0.01) (Figure 1). Dose response effects were seen (p<0.05). With respect to late afternoon/evening sleepiness (1700 to 1900 hour), each of the two split-dose regimens of modafinil produced significantly greater mean improvements from baseline in MWT sleep latency (p<0.05) than the 200-mg once-daily regimen (Figure 2, Panel A).
At baseline, only 11% (6 of 56) of all patients were able to remain awake for the first 20 minutes of the MWT sessions in the evening (Table 3). After 3 weeks of modafinil treatment, the percentage of patients across the 4 treatment groups who were able to sustain wakefulness for the first 20 minutes of the MWT evening sessions was significantly increased to 38% (21 of 56, p<0.05). A significantly higher proportion of patients receiving the modafinil 600-mg split-dose or the 400-mg split-dose regimens were able to sustain wakefulness for at least 20 minutes during both MWT sessions in the evening, compared with those receiving the 200-mg once-daily dose regimen (p<0.05) (Figure 2, Panel B).
At placebo-baseline, none of the study patients were able to sustain wakefulness for the first 20 minutes of the MWT sessions in the morning or afternoon. After 3 weeks of treatment, the modafinil 600-mg split-dose group had the highest proportion of patients able to sustain wakefulness for the first 20 minutes of the MWT sessions in the morning and afternoon, followed by the modafinil 400-mg split-dose, 400-mg once-daily, and 200-mg once-daily groups (Table 3).
After 3 weeks of treatment, all modafinil dosing regimens improved wakefulness, as demonstrated by reductions in mean ESS total scores from baseline. Significant improvement from baseline in mean ESS scores was observed in patients receiving the modafinil 200-mg (p<0.05) and 400-mg (p<0.0001) once-daily and 600-mg (p<0.05) split-dose regimens.
All four modafinil dosing regimens improved the overall clinical condition (CGI-C) after 3 weeks of treatment, relative to baseline (Figure 3). With respect to late-afternoon/evening sleepiness, the proportion of patients rated as “much improved” or “very much improved” was highest among those receiving the modafinil 600-mg and 400-mg split-dose regimens: 92% and 80%, respectively, compared with 70% of patients in the 400-mg once-daily group and 27% of patients in the 200-mg once-daily group. The proportion of patients rated as being at least “improved” was significantly higher in the modafinil 600-mg split-dose (100%), 400-mg split-dose (90%) and the 400-mg once-daily (91%) groups than in the 200-mg once-daily group (55%, p<0.01).
Safety Assessments
All modafinil dosing regimens were well tolerated. A total of 10 patients reported adverse events: seven receiving modafinil 400 mg once daily and one each receiving modafinil 200 mg once daily, modafinil 400 mg split dose, and 600 mg split dose. All adverse events were mild or moderate in severity. No serious adverse events were reported, and no patients discontinued the study because of adverse events. One patient receiving 400 mg once daily did experience adverse events (mild-to-moderate agitation, irritability, nervousness, anxiety, gastrointestinal distress, and insomnia) that led to temporary discontinuation of modafinil; however, the patient continued with the established protocol. The most common adverse events assessed by the investigator(s) as being possibly or probably related to treatment were gastrointestinal events (nausea and dyspepsia), which occurred in a total of three patients (5%) who received once-daily regimens of modafinil 200 mg or 400 mg. Other adverse events potentially related to modafinil were headache (N=1) and emotional lability (N=1), which occurred with the 400-mg once-daily regimen, and anxiety (N=1), which occurred with the 200-mg once-daily regimen (overall incidence of 2% for each adverse event). Results of laboratory tests, physical examinations, vital sign measurements, and ECG recordings indicated no clinically significant treatment-related abnormalities.
DISCUSSION
The investigations included in this analysis were the first to directly compare the efficacy of once-daily and split-dose regimens of modafinil in improving wakefulness throughout the entire waking day in patients with narcolepsy who experienced a positive response to modafinil early in the day but had subsequent late-day sleepiness. The findings of the MWT showed that 600 mg of modafinil administered as a split dose and 400 mg administered either once daily or as a split dose were significantly more effective in maintaining wakefulness throughout the day than 200 mg once daily in this population. Significant improvements in clinical condition (CGI) with respect to evening sleepiness further demonstrated that the higher once-daily dose and split-dose regimens were significantly better than the 200-mg once-daily dose in facilitating wakefulness throughout the day. All four dosage regimens of modafinil (200 mg once-daily, 400 mg once-daily, 400 mg split-dose, and 600 mg split-dose) significantly improved daytime wakefulness and overall clinical condition compared with baseline. Modafinil was generally well tolerated, with no apparent differences in the frequency or severity of adverse events among dosing regimens and no treatment discontinuations because of adverse events.
Although once-daily and split-dose modafinil regimens have not been directly compared in previous clinical trials, the efficacy of different modafinil doses and dosing regimens has been previously evaluated. These findings suggest that 400 mg once daily is superior to 200 mg once daily in maintaining wakefulness throughout the day. In contrast, in two large-scale clinical trials, no statistical difference in efficacy was found between 200-mg and 400-mg modafinil once-daily doses.1,2 However, the latter studies included a different study population, were not designed to assess late-day wakefulness, and employed distinct testing procedures. The previous trials included modafinil-naive patients,1,2 whereas the present study included patients who were stabilized on higher mean doses of modafinil (approximately 368 mg/day). In these previous trials, modafinil was administered approximately 1 hour prior to the first MWT session, and MWT assessments were discontinued in the early afternoon. As modafinil serum levels peak 2 to 4 hours after administration, the testing schedule used in the present study protocol offered a greater opportunity to discriminate between the effects of the 200-mg and 400-mg doses over a longer time period. By using an extended MWT protocol, we were able to capture differences in response between the tested doses and dose regimens in the evening (1700–1900 hour). Finally, the studies included in this analysis employed 30-minute rather than 20-minute MWT sessions, thus minimizing the potential for a ceiling effect.16
In the present study, we found significant improvement in late-afternoon and evening sleepiness with both the 400-mg and 600-mg split-dose regimens of modafinil. Broughton et al. demonstrated dose-related differences in efficacy between a 200-mg split-dose and a 400-mg split-dose regimen.3 In their study, which used a 40-minute MWT session, the 400-mg split dose significantly improved MWT sleep latency at all testing times (i.e., 0930, 1130, 1330, and 1530 hour), whereas the 200-mg split dose significantly improved sleep latency only at the two testing sessions following the midday dose (i.e., 1330 and 1530 hour). Split-dose regimens of modafinil have also been evaluated in two crossover trials,8,9 but dose-response differences were not assessed. Patients receiving split-dose modafinil dosing regimens in these trials8,9 (300 mg/day and 300 mg–500 mg, respectively) demonstrated significant improvement in wakefulness, as assessed by MWT, the ESS, and patient diaries, without adverse effects on nighttime sleep. Similar to our findings, Billiard et al.9 reported that modafinil 300 mg as a split dose reduced excessive daytime sleepiness, measured by sleep latency on MWT, in the morning (1000 hour) through the early evening (1800 hour).
The results of the present study may have important implications for optimal dosing of modafinil, but several limitations should be considered. Although significant improvements from baseline were demonstrated in objective measures of wakefulness and overall clinical condition for all dosage regimens of modafinil, the small sample size for each treatment group (N≤23) may have limited the detection of treatment differences. Thus, these findings need to be confirmed in controlled trials using larger sample sizes. In addition, the studies included only narcolepsy patients with documented clinical response to modafinil. Because a complex set of genetic, immunological, and environmental factors may influence a patient’s responsiveness to modafinil,17 the magnitude of the clinical response observed in our study may be exaggerated vis-à-vis the narcolepsy patient population at large. Moreover, as the study included only patients experiencing late-afternoon/evening sleepiness despite satisfactory earlier daytime responses to modafinil, the observed time-of-day effects may not be reproducible in all narcolepsy patients. Nonetheless, the demonstrated advantages of various dose and dose regimens of modafinil for prolonging wakefulness throughout the day may apply to patients exhibiting a treatment response pattern similar to that of the patients in the study sample. Finally, the present studies did not include nocturnal polysomnography or subjective estimates of sleep and sleep quality to evaluate the potential effects of the modafinil dosing regimens on sleep. Therefore, we cannot verify from these studies that the dosing regimens did not interfere with nocturnal sleep. Other studies, however, have reported that 200-mg and 400-mg once-daily doses1,2 and 200-mg and 400-mg split doses3 of modafinil did not effect objectively recorded sleep.
Given the emergence of late-day sleepiness in some narcolepsy patients treated with the approved once-daily dose of modafinil, it is apparent that the pharmacokinetics of this agent do not always correlate with its pharmacodynamic effects. In such patients, a twice-daily dose regimen may be more appropriate than the traditional once-daily dose regimen. This pooled analysis revealed that addition of a 200-mg midday dose of modafinil to either 200-mg or 400-mg morning doses is well tolerated and effectively sustained wakefulness throughout the entire waking day in narcolepsy patients who experienced late-afternoon or evening sleepiness with once-daily dose regimens of modafinil.
ACKNOWLEDGMENTS
This study was supported by Cephalon, Inc.
Dr. Schwartz is a consultant to Cephalon Inc., and Dr. Feldman is part of the Cephalon Inc. Speakers Bureau.
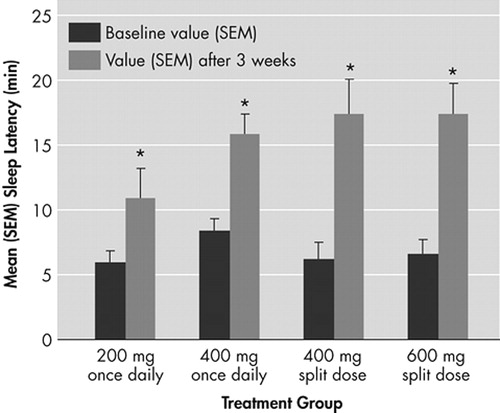
FIGURE 1. Mean (SEM) Sleep Latency Times for the First Six Sessions of the Maintenance of Wakefulness Testing (MWT) (0900 hour to 1900 hour) at Baseline and After 3 Weeks of Modafinil Treatment, According to Dosing Regimen
*p<0.01 for change from baseline
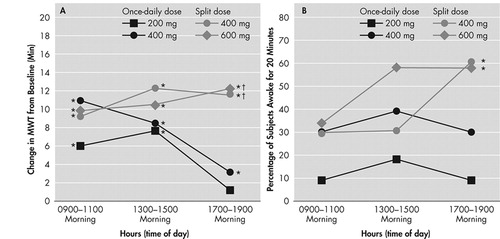
FIGURE 2.
Panel A: Mean (SEM) Change From Baseline in Maintenance of Wakefulness Test (MWT) Sleep Latency Times for the Morning (0900 to 1100 hour), Afternoon (1300 to 1500 hour), and Evening (1700 to 1900 hour) Sessions for Each Modafinil Treatment Regimen
*p<0.01 for change from baseline for each group; †p<0.05 for modafinil 400 mg split dose (0700 and 1200 hour) and modafinil 600 mg split dose versus modafinil 200 mg once daily (at 0700 hour)
Panel B: Percentage of Patients Remaining Awake for the First 20 Minutes of Both Maintenance of Wakefulness Test (MWT) Sessions in the Morning, Afternoon, and Evening for Each Modafinil Treatment Group
*p<0.05 for modafinil 400 mg split dose and modafinil 600 mg split dose versus modafinil 200 mg once daily
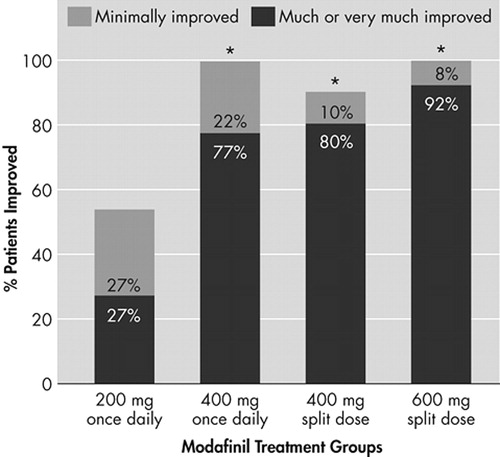
FIGURE 3. Percentages of Patients Whose Clinical Conditions Improved After 3 Weeks of Modafinil Treatment, As Measured With the CGI-C Scale
*p<0.01 for between-group comparison with modafinil 200 mg once daily
 |
 |
 |
1 US Modafinil in Narcolepsy Multicenter Study Group: Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann Neurol 1998; 43:88–97Crossref, Medline, Google Scholar
2 US Modafinil in Narcolepsy Multicenter Study Group: Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology 2000; 54:1166–1175Crossref, Medline, Google Scholar
3 Broughton RJ, Fleming JA, George CF, et al: Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurol 1997; 49:444–451Crossref, Medline, Google Scholar
4 Scammell TE, Estabrooke IV, McCarthy MT, et al: Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000; 20:8620–8628Crossref, Medline, Google Scholar
5 Lin JS, Hou Y, Jouvet M: Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci USA 1996; 93:14128–14133Crossref, Medline, Google Scholar
6 Chemelli RM, Willi JT, Sinton CM, et al: Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98:437–451Crossref, Medline, Google Scholar
7 Robertson P, Hellriegel ET: Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet 2003; 42:123–137Crossref, Medline, Google Scholar
8 Moldofsky H, Broughton RJ, Hill JD: A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy. Sleep Med 2000; 1:109–116Crossref, Medline, Google Scholar
9 Billiard M, Besset A, Montplaisir J, et al: Modafinil: a double-blind multicentric study. Sleep 1994; 17:S107-S112Google Scholar
10 American Sleep Disorders Association: The International Classification of Sleep Disorders: Diagnostic and Coding Manual. Lawrence, Kan, Allen Press Inc, 1997Google Scholar
11 Guy W: ECDEU Assessment Manual for Psychopharmacology. Washington, DC, National Institute of Mental Health, US Dept of Health, Education, and Welfare, 1976Google Scholar
12 Schwartz JRL, Feldman NT, Bogan RK, et al: Dosing-regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy. Clin Neuropharmacol 2003; 26:252–257Crossref, Medline, Google Scholar
13 Mitler MM, Gujavarty KS, Browman CP: Maintenance of Wakefulness Test: a polysomnographic technique for evaluation of treatment efficacy in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol 1982; 53:658–661Crossref, Medline, Google Scholar
14 Johns MW: A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991; 14:540–545Crossref, Medline, Google Scholar
15 Huitema BE: Analysis of Covariance and Alternatives. New York, NY, John Wiley & Sons, 1980Google Scholar
16 Schecter-Amir D, Wade J, Moldofsky H: Narcolepsy and the ability to resist sleep. Sleep Med 2000; 1:101–108Crossref, Medline, Google Scholar
17 Dauvilliers Y, Neidhart E, Billiard M, et al: Sexual dimorphism of the catechol-O-methyltransferase gene in narcolepsy is associated with response to modafinil. Pharmacogenomics J 2002; 2:65–68Crossref, Medline, Google Scholar



