Blast-Related Traumatic Brain Injury: What Is Known?
Historical accounts note that as more powerful explosives came into general use in warfare, a condition was described in which soldiers were rendered dazed or unconscious by an explosion that caused no external visible injury. Retrograde and anterograde amnesia were commonly present upon regaining consciousness or awareness, as were severe headache, tinnitus, hypersensitivity to noise, and tremors. 6 – 9
During World War I, there was significant interest in studying and reporting about cases of soldiers who survived blast exposure and developed neuropsychiatric symptoms. Drs. Fred Mott and Gordon Holmes, two famous physicians with the British Army, wrote in great detail about their battle-front hospital experiences. 6 , 7 , 10 , 11 They attempted to describe the neurological/psychiatric status of soldiers post combat. A variety of names were used for this condition, including commotio cerebri , shell shock, and functional neurosis. These early authors had difficulty differentiating physical injury to the brain from emotional trauma. Thus, these terms were often used loosely to describe what would in the year 2006 be diagnosed as posttraumatic delirium or agitation, concussion, acute stress syndrome, posttraumatic stress disorder, psychosis, or conversion disorders. Mott separated these into two groups. For those that were buried by the explosion, he believed the overriding injury to the brain was carbon monoxide poisoning. In the absence of burial or external injury, Mott believed that the condition was purely due to “psychic trauma” or emotional distress. 6 , 7 , 10 , 12 The controversy regarding physical versus emotional cause for “shell shock” (often in this time referred to as cerebral blast syndrome and cerebral blast concussion) continued into World War II. While some physicians were convinced that there was no “organic” injury to the brain, others reported EEG changes similar to those from confirmed closed head injury. 8 , 9
Blast-Related Forces
The changes in atmospheric pressure that cause primary blast injuries arise because a high-explosive detonation results from the nearly instantaneous conversion of a solid or liquid into gasses. 1 – 3 Momentarily these gasses occupy the same volume as the parent solid or liquid and thus they are under extremely high pressure. The gasses expand rapidly, causing compression in the surrounding air, forming a pulse of pressure (blast overpressure, positive phase of the blast wave) ( Figure 1 ). As the gasses continue to expand, the pressure drops, creating a relative vacuum (blast underpressure, negative phase of the blast wave). Extreme pressure differences occur as the blast wave reaches the body, resulting in both stress and shear waves.
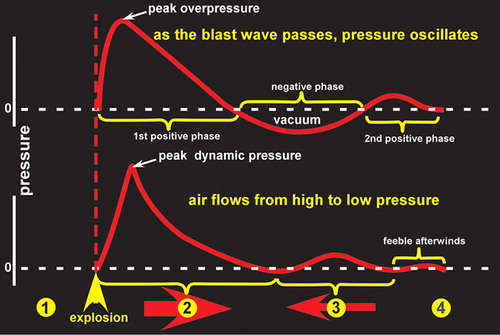
Primary blast injury results from blast wave-induced changes in atmospheric pressure (barotrauma). Organs and tissues of different densities are accelerated at different relative rates, resulting in displacement, stretching and shearing forces. The most vulnerable parts of the body to primary blast injury are considered to be those with air-fluid interfaces, particularly the lungs, bowel, and middle ear. Rupture of the tympanic membrane is the most frequent injury. Both the blast wave and blast wind can propel objects with considerable force, causing secondary and tertiary blast injuries. Secondary blast injury results from objects put in motion by the blast wind impacting a person (ballistic trauma). This category includes both injuries due to flying debris and due to collapse of structures. Tertiary blast injury results from a person being blown into solid objects by the blast wind.
Blast-Related Brain Injury
The brain is clearly vulnerable to both secondary and tertiary blast injury. A still unresolved controversy is whether primary blast forces directly injure the brain. Shear and stress waves from the primary blast could potentially cause traumatic brain injury (TBI) directly (e.g., concussion, hemorrhage, edema, diffuse axonal injury). The primary blast can also cause formation of gas emboli, leading to infarction. 13
Clinical data for brain injury due to primary blast forces is quite limited. Most studies involve war-related injuries, although blast-related injury due to air blast and firecrackers has also been reported. In a battlefield situation, it can be extremely difficult to confidently identify cases in which only primary blast injury is present. In addition, neuropathological information was only occasionally available. Almost a century of medical literature provides a handful of cases in which brain injuries are likely to have resulted from primary blast forces. 9 , 12 , 14 – 17 Reported neuropathological changes have included small hemorrhages within white matter, chromatolytic changes in neurons (due to degeneration of Nissl bodies, an indication of neuronal damage), diffuse brain injury and subdural hemorrhage. Mott wrote in detail about a few cases where primary blast was the proposed cause of death. 12 He noted a variety of microscopic findings including an “extremely congested” cortex, perivascular space enlargement, subpial hemorrhages, venous engorgement, white matter hemorrhage into the myelin sheath and perivascular spaces, and chromatolysis. Although Mott believed that most cases of “shell-shock” resulted from “psychic trauma”, he was aware that available methods for examining the brain were quite limited.
“So complex is the structure of the human CNS, and so subtile the chemical and physical changes underlying its functions, that because our gross methods of investigating dead material do not enable us to say that the living matter is altered.…” 6
The truth of this caution today, though written more than 90 years ago, has recently been reinforced by finding from experimental (animal) studies of TBI. 18 It has been shown that severely injured axons do not necessarily swell. The presence of focal axonal swellings, the most commonly used neuropathological marker for TBI, may therefore seriously underestimate the magnitude of injury present. The authors also noted that the insensitivity of presently used neuropathological markers to the unmyelinated fine caliber axons that make up ∼ 30% of corpus callosum may also contribute to inaccurate injury evaluation. Thus, the search for better methods of delineating the microscopic aspects of brain injury continues.
The vulnerability of the brain to primary blast forces is supported by recent animal studies. One group examined the effects of exposure to pure primary blast forces by enclosing their subjects (rats) within a concrete bunker to prevent injury by other mechanisms. They reported widespread microglial activation (a hallmark of neural degeneration), particularly in the superficial layers of the cerebral and cerebellar cortices. 19 Other areas, such as the pineal gland, were also affected. 20 In a later study, performance on tests of coordination, balance, and strength was significantly impaired by exposure to a 20 kPa (kPa is a measure of pressure) explosion but not by exposure to a 2.8 kPa explosion. 21 More degenerating neurons were seen in cerebral cortex following the larger explosion.
Another group (utilizing rabbits and rats) studied the effect on the brain of exposure to primary blast (delivered by a shock tube) sufficient to cause a moderate level of lung injury. 22 – 24 Special holders were used to prevent occurrence of secondary and tertiary blast injuries. In some cases, the effect of exposing the whole body was compared to exposing only the thoracic region (with the head protected by a steel plate). 23 Both types of blast exposure resulted in ultrastructural evidence of neuronal injury (expanded perineuronal spaces, cytoplasmic vacuoles, myelin deformation, axoplasmic shrinkage) in the areas examined (hippocampus, brainstem reticular formation). The authors noted that the pattern of neuronal abnormalities is similar to those seen in diffuse axonal injury. Biochemical changes indicative of oxidative stress were also present. The degree of neuronal damage correlated with impaired performance on an active avoidance task.
Other groups have reported the effects of exposure to blast forces at levels designed to be below the threshold for induction of macroscopic lung injury. 25 , 26 One reported a change in localization of neurofilament protein staining from axons and dendrites to the cell body in cortical neurons following exposure (of rats). 25 The authors noted that these changes are likely the result of disturbed anterograde axonal transport. Another group used an open air exposure (utilizing pigs oriented with their hind quarters closest to the explosive) just below the threshold for induction of macroscopic lung injury. 26 Depression of electroencephalographic (EEG) activity was reported in one-half the animals immediately following the blast exposure. EEG amplitude was reduced by about 50% for a short time (5 – 15 sec), with a return of normal activity within 1–2 minutes.
Traumatic Brain Injury
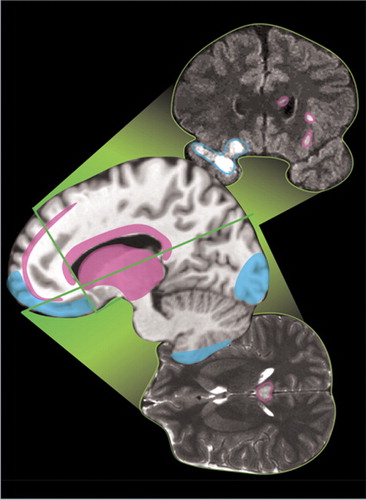
The most common types of TBI are diffuse axonal injury, contusion, and subdural hemorrhage ( Figure 2 ). 27 Diffuse axonal injuries are very common following closed head injuries. They result when shearing, stretching, and/or angular forces pull on axons and small vessels. 28 Impaired axonal transport leads to focal axonal swelling and (after several hours) may result in axonal disconnection. 29 The most common locations are the corticomedullary (gray matter-white matter) junction (particularly in the frontal and temporal areas), internal capsule, deep gray matter, upper brainstem, and corpus callosum ( Figure 2 , pink). 27 Magnetic resonance imaging (MRI) is more sensitive than computed tomography (CT) in detecting diffuse axonal injury. 27 , 29 T2 weighted magnetic resonance (MR) images, especially fluid attenuated inversion recovery (FLAIR) images, are best for visualizing nonhemorrhagic lesions. Some studies indicate that diffusion weighted MR may be even more sensitive than T2 weighted for identifying edema. 30 Gradient echo MR images are more sensitive to areas of hemorrhage. 30 , 31
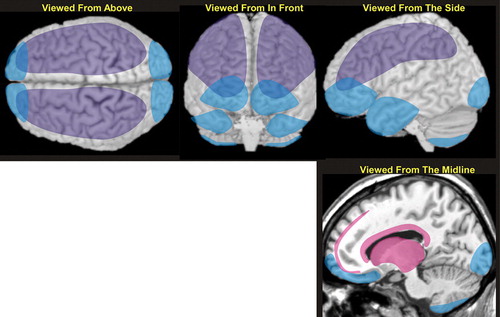
Contusions occur when the brain moves within the skull enough to impact bone, causing bruising of the brain parenchyma (hemorrhage and edema). The most common locations are the superficial gray matter of the inferior, lateral and anterior aspects of the frontal and temporal lobes, with the occipital poles or cerebellum less often involved ( Figure 2 , blue). 27 The imaging appearance of contusion is variable. 27 Edema has lower signal intensity than brain on CT. In the absence of hemorrhage, CT may initially be only minimally abnormal. If hemorrhage is present, there are commonly multiple bright areas of variable size. Edema appears bright on T2 weighted or FLAIR MRI. The most common sequence in the appearance of hemorrhage on T2 weighted MRI is from bright initially, to mildly strongly dark within the first 2–3 days, to bright again by 2–3 weeks. 32 Small areas of hemorrhage may be most easily identified with gradient echo MR. Progression is common, with 25% demonstrating delayed hemorrhage over the initial 48 hours. 33
Traumatic subdural hemorrhage occurs when the brain moves within the skull enough to tear the tributary surface veins that bridge from the brain surface to the dural venous sinus. 32 The most common locations are the frontal and parietal convexities on the same side as the injury ( Figure 2 , purple). 27 The usual imaging appearance of subdural hemorrhage is an extraaxial, crescent-shaped, homogeneous fluid collection that conforms to the cerebral surface. 27 , 32 Its spread is limited by the dural reflections, and it rarely crosses the midline. Collections greater than 5 mm are easily recognized. Smaller collections may be missed due to partial volume effects with adjacent bone. Acute subdural hemorrhage is usually hyperintense on CT, the preferred imaging modality to evaluate for hemorrhage. It may be of mixed intensity or isointense to gray matter in patients with anemia. In these cases, it can be identified by its mass effects including sulcal effacement, inward buckling of the gray-white interface, and presence of midline shift.
Conclusion
The potential neuropsychiatric implications of such widespread exposure to blast are still uncertain. However, the Defense and Veterans Brain Injury Center (DVBIC) has reported that 59% of an “at risk” group of injured soldiers returning from Afghanistan or Iraq to Walter Reed (2003–2004) suffered at least a mild TBI while in combat. 34 , 35 Further characterization of 433 war fighters revealed that the TBI was moderate or severe in more than half the group. The TBI was due to a closed head injury in 88%. Similarly, a study of patients with explosive injury only to the lower extremities found that 51% (665/1303) had neurological symptoms (e.g., headache, insomnia, psychomotor agitation, vertigo) consistent with TBI. 36 Of these, 36% had EEG alterations during the acute stage (most commonly hypersynchronous, discontinuous, or irregular brain activity). Both neurological and EEG abnormalities persisted into the chronic stage for 30% of this group. An earlier study found that veterans with post traumatic stress disorder who had been exposed to blast had EEG abnormalities and attentional difficulties consistent with mild TBI. 37 Thus, the limited clinical evidence to date suggests a similar range of neuropsychiatric impairments as seen with other traumas (e.g., accidents, assaults). In many cases, TBI clearly resulted from secondary and/or tertiary blast injuries. The vulnerability of the human brain to primary blast injury is controversial and an area of active research. 38
1. Mayorga MA: The pathology of primary blast overpressure injury. Toxicology 1997; 121:17–28Google Scholar
2. Wightman JM, Gladish SL: Explosions and blasts injuries. Ann Emerg Med 2001; 37:664–678Google Scholar
3. DePalma RG, Burris DG, Champion HR et al: Blast injuries. N Engl J Med 2005; 352:1335–1342Google Scholar
4. Murray CK, Reynolds JC, Schroeder JM et al: Spectrum of care provided at an Echelon II medical unit during Operation Iraqi Freedom. Mil Med 2005; 170:516–520Google Scholar
5. Gondusky JS, Reiter MP: Protecting military convoys in Iraq: an examination of battle injuries sustained by a Mechanized Battalion during Operation Iraqi Freedom II. Mil Med 2005; 170:546–549Google Scholar
6. Mott FW: The effects of high explosives upon the CNS. Lecture I. Lancet 1916; 4824:331–338Google Scholar
7. Mott FW: The effects of high explosives upon the CNS. Lecture III. Lancet 1916; 4828:545–553Google Scholar
8. Fabing HD: Cerebral blast syndrome in combat soldiers. Arch Neurol Psychiatry 1947; 57:14–57Google Scholar
9. Cramer F, Paster S, Stephenson C: Cerebral injuries due to explosion waves—“cerebral blast concussion.” Arch Neurol Psychiatry 1949; 61:1–20Google Scholar
10. Mott FW: The effects of high explosives upon the CNS. Lecture II. Lancet 1916; 4826:441–449Google Scholar
11. Macleod AD: Shell shock, Gordon Holmes and the great war. J R Soc Med 2004; 97:89Google Scholar
12. Mott FW: The microscopic examination of the brains of two men dead of commotio cerebri (shell shock) without visible external injury. J R Army Med Corps 1917; 29:662–677Google Scholar
13. Guy RJ, Glover MACNP: Primary blast injury: pathophysiology and implications for treatment. Part 3: Injury to the CNS and the limbs. J R Nav Med Serv 2000; 86:27–31Google Scholar
14. Sylvia FR, Drake AI, Wester DC: Transient vestibular balance dysfunction after primary blast injury. Mil Med 2001; 66:918–920Google Scholar
15. Murthy JMK, Chopra JS, Gulati DR, et al: Subdural hematoma in an adult following a blast injury. J Neurosurg 1979; 50:260–261Google Scholar
16. Hirsch AE, Ommaya AK: Head injury caused by underwater explosion of a firecracker. J Neurosurg 1972; 37:95–99Google Scholar
17. Levi L, Borovich B, Guilburd JN et al: Wartime neurosurgical experience in Lebanon, 1982–85. II. Closed craniocerebral injuries. Isr J Med Sci 1990; 26:555–558Google Scholar
18. Povlishock JT, Katz DI: Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005; 20:76–94Google Scholar
19. Kaur C, Singh J, Lim MK, et al: The response of neurons and microglia to blast injury in the rat brain. Neuropathol App Neurobiol 1995; 21:369–377Google Scholar
20. Kaur C, Singh J, Lim MK, et al: Macrophages/microglia as “sensor” of injury in the pineal gland of rats following a nonpenetrative blast. Neurosci Res 1997; 27:317–322Google Scholar
21. Moochhala SM, M.D. S, Lu J,et al Neuroprotective role of aminoguanidine in behavioral changes after blast injury. J Trauma 2004; 56: 393–403Google Scholar
22. Cernak I, Savic J, Malicevic Z, et al: Involvement of the CNS in the general response to pulmonary blast injury. J Trauma 1996; 40:100–104Google Scholar
23. Cernak I, Wang Z, Jiang J, et al: Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma 2001; 50:695–706Google Scholar
24. Cernak I, Wang Z, Jiang J, et al: Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj 2001; 15:593–612Google Scholar
25. Saljo A, Bao F, Haglid KG, et al: Blast exposure causes redistribution of phosphorylated neuofilament subunits in neurons of the adult rat brain. J Neurotrauma 2000; 17:719–726Google Scholar
26. Axelsson H, Hjelmqvist H, Medin A, et al: Physiological changes in pigs exposed to a blast wave from a detonating high-explosive charge. Mil Med 2000; 165:119–126Google Scholar
27. Gutierrez-Cadavid JE: Imaging of head trauma, in Imaging of the Nervous System. Edited by Latchaw RE, Kucharczyk J, Moseley ME. Elsevier Mosby, Philadelphia, 2005, pp 869–904Google Scholar
28. Mendez CV, Hurley RA, Lassonde M et al: Mild traumatic brain injury: Neuroimaging of sports-related concussion. J Neuropsychiatry Clin Neurosci 2005; 17:297–303Google Scholar
29. Hurley RA, McGowan JC, Arfanakis K, et al: Traumatic axonal injury: Novel insights into evolution and identification. J Neuropsychiatry Clin Neurosci 2004; 16:1–7Google Scholar
30. Huisman TAGM, Sorensen AG, Hergan K, et al: Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr 2003; 27:5–11Google Scholar
31. Tong KA, Ashwal S, Shutter LA et al: Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 2003; 227:332–339Google Scholar
32. Taber KH, Hayman LA, Diaz-Marchan PJ, Rauch RA: Imaging of intracranial blood, in Imaging of the nervous system. Edited by Latchaw RE, Kucharczyk J, Moseley ME. Elsevier Mosby, Philadelphia,2005; 555–575Google Scholar
33. Orrison WW, Moore KR: Neuroimaging and Head Trauma, in Neuroimaging. Edited by Orrison WW. W.B. Saunders Company, Philadelphia, 2000, pp 885–915Google Scholar
34. Okie S: Traumatic Brain Injury in the War Zone. N Engl J Med 2005; 352:2043–2047Google Scholar
35. Warden DL, Ryan LM, Helmick KM, et al: War neurotrauma: the Defense and Veterans Brain Injury Center (DVBIC) experience at Walter Reed Army Medical Center (WRAMC). J Neurotrauma 2005; 22:1178Google Scholar
36. Cernak I, Savic J, Ignjatovic D, et al: Blast injury from explosive munitions. J Trauma 1999; 47:96–103Google Scholar
37. Trudeau DL, Anderson J, Hansen LM et al: Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. J Neuropsychiatry Clin Neurosci 1998; 10:308–313Google Scholar
38. Cernak I: Blast (explosion)-induced neurotrauma: A myth becomes reality. Restorative Neurology and Neuroscience 2005; 23:139–140Google Scholar



