White Matter Changes Associated With Psychotic Symptoms in Alzheimer’s Disease Patients
Presently, only limited numbers of MRI or CT studies have examined the association of white matter changes with the neuropsychiatric manifestations of Alzheimer’s disease, and there is a lack of agreement among the results of these studies. Some studies have shown a significant association between white matter changes and certain neuropsychiatric symptoms, such as depression, 6 apathy, 7 suicidal ideation or low self-esteem, 8 and aberrant motor behavior, 9 whereas other studies have failed to find any relationship in Alzheimer’s disease patients. 10 , 11 No previous study on white matter changes in Alzheimer’s disease has indicated a relationship between psychotic symptoms and white matter changes, though a CT study conducted for Alzheimer’s disease patients suggested a relationship between delusion and lacunar infarcts of white matter. 12 Psychotic symptoms also have been observed in many white matter disorders, such as adult-onset metachromatic leukodystrophy, 13 , 14 multiple sclerosis, 15 Binswanger’s disease, 16 and normal pressure hydrocephalus. 17 , 18 These discrepancies may be related to the differences not only in study population but in definition and quantification method of white matter changes or assessment method of neuropsychiatric symptoms.
Most previous studies on white matter changes of Alzheimer’s disease have examined only the relationship between global white matter changes and neuropsychiatric symptoms without exploring further the effect of regional white matter changes, 7 , 9 – 11 though specific focal changes of cerebral white matter may contribute to the development of particular neuropsychiatric symptoms.
This study aimed to examine the relationship between white matter changes seen on MRI and neuropsychiatric symptoms in probable Alzheimer’s disease patients. We tried to explore the association of brain region-specific white matter changes and global white matter changes with those symptoms.
METHOD
Subjects
Subjects for this study were recruited from a cohort of Alzheimer’s disease patients who regularly follow up at the Dementia & Age-Associated Cognitive Decline Clinic of Seoul National University Hospital in Seoul, Korea. All candidate patients were examined by psychiatrists with advanced training in neuropsychiatry and dementia research according to the protocol of the Korean Version of the Consortium to Establish a Registry for Alzheimer’ disease Assessment Packet (CERAD-K). 19 , 20 Reliable informants were interviewed to acquire accurate information regarding the cognitive and functional changes and medical history of the subjects. Psychiatric, general physical and neurological examinations, and routine laboratory tests were performed. After reviewing all of the available raw data, a panel of four psychiatrists with expertise in dementia research made the clinical decisions, including clinical diagnosis, clinical dementia rating (CDR), 21 and scoring for modified Hachinski Ischemic Scale (mHIS). 22 All the subjects included in this study met both the Diagnostic and Statistical Manual of Mental Disorders (DSM–IV) criteria for dementia 23 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’ disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable Alzheimer’s disease. 24
Exclusion criteria consisted of any serious medical, psychiatric, and neurological disorders that could affect mental function; evidence of focal brain lesions on MRI, including lacunar infarcts and hematoma; presence of severe behavioral or communication problems that would make clinical or MRI examination difficult; and absence of a reliable informant. The Institutional Review Board of Seoul National University Hospital approved the study protocol and informed consent was obtained from all of the subjects and their relatives.
Assessment of Cognitive Functions and Neuropsychiatric Symptoms
We assessed the cognitive function of the subjects with eight neuropsychological tests (verbal fluency, animal category; 15-item Boston naming test; Mini-Mental State Examination; word list memory; word list recall; word list recognition; constructional praxis; constructional recall) included in CERAD-K. 19 The subjects’ neuropsychiatric symptoms were assessed during an interview with the informant by using CERAD Behavioral Rating Scale for Dementia (BRSD). 25 The BRSD includes 46 items on the behavioral problems and psychiatric symptoms of dementia patients and each is scaled by frequency of occurrence. Severity is also reflected in the BRSD in two ways: first, a number of items are followed by a probe, and the score is adjusted according to the response to the probe; and second, a number of items are combined to generate six factor-based subscale scores (Depressive Symptoms, Inertia, Vegetative Symptoms, Irritability/Aggression, Behavioral Dysregulation, and Psychotic Symptoms), so that the more items that are rated present on a given subscale, the greater the severity of the construct represented by the subscale. 26
MRI Acquisition and Assessment of White Matter Changes
MRI was performed using General Electronics 1.5-tesla SIGNA Scanner (GE Medical Systems, Milwaukee, U.S.). Spin-echo pulse sequences were used to generate a series of 15 T2-weighted images (repetition time = 4,500 msec, echo time = 99 msec, number of excitations = 1), which then were used to assess white matter changes. These were complemented by either T1-weighted (repetition time = 500 msec, echo time = 12 msec, number of excitations = 1) or fluid-attenuated inversion recovery (FLAIR) (repetition time = 10,002 msec, echo time = 133 msec, inversion time = 2,200 msec, number of excitations = 1) images to discriminate white matter changes from lacunar infarcts. In all image acquisitions, the field of view was 210×210 mm, and the matrix size was 256×256.
White matter changes on MRI were classified as ill-defined hyperintensities ≥ 5 mm on T2-weighted images. Lacunar infarcts were classified as well-defined lesions with a diameter of > 2 mm with hyperintensity on T2-weighted images and the hypointensity on T1-weighted or FLAIR images. If lesions with this characteristic were ≤ 2 mm, they were considered as perivascular spaces, except around the anterior commissure, where perivascular spaces can be large. Changes in the basal ganglia were rated in the same way and considered white matter lesions even if located in the gray matter nuclei, which contains a small amount of white matter.
White matter changes were assessed by a scale proposed by the European Task Force on Age-Related White Matter Changes. 27 Eight different regions were rated separately: left and right frontal areas (the frontal lobes anterior to the central sulcus); left and right parieto-occipital areas (the parietal and occipital lobes together); left and right temporal areas (the border between the parieto-occipital and temporal lobes was approximated as a line drawn from the posterior part of the Sylvian fissure to the trigone areas of the lateral ventricles); and left and right basal ganglia (the striatum, globus pallidus, thalamus, internal and external capsules, and insula). Rating scores for white matter changes of frontal, parieto-occipital, and temporal areas were designated as follows: 0 = no lesions including symmetrical, well-defined caps or bands; 1 = focal lesions; 2 = beginning confluence of lesions, and 3 = diffuse involvement of the entire region, with or without involvement of U fibers. Scores for basal ganglia lesions were as follows: 0 = no lesions; 1 = 1 focal lesion ≥5 mm; 2 = >1 focal lesion; and 3 = confluent lesions. The overall score for white matter changes was calculated by adding up the eight regions’ scores. The interrater reliability between two psychiatrists for the rating of each region was examined with 20 randomly selected patients, and good kappa coefficients were obtained: 0.77 for left frontal, 0.70 for right frontal, 0.73 for left parieto-occipital, 0.64 for right parieto-occipital, 0.58 for left temporal, 0.57 for right temporal, 0.69 for left basal ganglia, 0.62 for right basal ganglia. Final white matter changes ratings were carried out by one psychiatrist who had no knowledge of the subjects’ clinical data, including cognitive test results and BRSD scores.
Statistical Analysis
Nonparametric statistical analyses were performed because the scores of white matter changes were assessed as ordinal scales. All correlations were examined by using partial Spearman rank correlation coefficients after controlling for the effects of age, education, sex, and duration of illness, which have been associated with neuropsychiatric symptoms, 28 , 29 cognitive dysfunctions 30 and white matter changes 31 in Alzheimer’s disease. For all analyses, α level was set at 0.05. We did not make any adjustment for multiple comparisons because we tried to explore only the relationship between neuropsychiatric manifestations and white matter changes with no specific hypotheses to be tested. All statistical analyses were carried out with the SAS program, version 6.12 (Statistical Analysis Systems).
RESULTS
Subjects’ Characteristics
The subjects comprised 37 women and 18 men; the mean standard deviation age at evaluation was 69.7 (SD = 8.9 years), and the mean educational attainment was 7.9 (SD = 5.8 years). The mean duration of illness was 3.1 (SD = 1.8) years, and the mean score of mHIS was 0.3 (SD = 0.5). The functional severity was “very mild” in 14 subjects (25.5%), “mild” in 23 subjects (41.8%), “moderate” in 15 subjects (27.3%), and “severe” in three subjects (5.5%), as determined by the CDR. The numbers (proportions) of subjects with specific neuropsychiatric symptoms, categorized by BRSD subscales, were as follows: depression was observed in 38 subjects (69.1%); inertia in 37 subjects (67.3%); irritability in 37 subjects (67.3%); behavioral dysregulation in 24 subjects (43.6%); vegetative symptoms in 25 subjects (45.5%); and psychotic symptoms in 10 subjects (18.2%). When a BRSD subscale score was greater than zero, we determined that the neuropsychiatric symptom related to the subscale existed. The mean standard deviation of each neuropsychological test was: verbal fluency, 6.7 (SD = 3.4); 15-item Boston Naming Test, 5.8 (SD = 3.1); Mini-Mental State Examination, 15.6 (SD = 5.9); word list memory, 6.2 (SD = 4.1); word list recall, 0.8 (SD = 1.3); word list recognition, 3.2 (SD = 2.5); constructional praxis, 7.6 (SD = 2.6); constructional recall, 0.8 (SD = 1.3).
Associations Among Overall White Matter Changes, Neuropsychiatric Symptoms, and Cognitive Test Performances
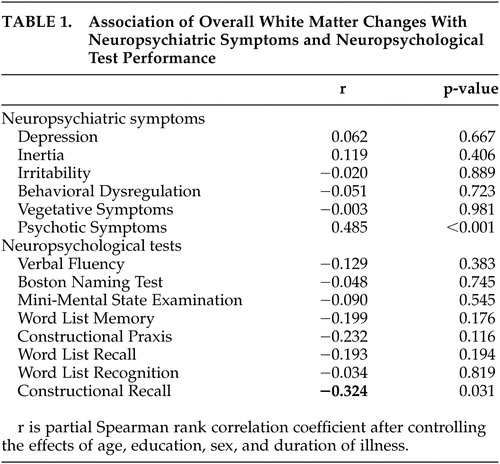
As shown in Table 1 , overall white matter changes significantly correlated only with the score of the Psychotic Symptoms subscale, after controlling for the effects of age, sex, education, and duration of illness. No significant correlation was noted between the overall white matter changes and the scores of other BRSD subscales. White matter changes significantly correlated with constructional recall performance, while there was no significant correlation observed between any other cognitive test score and overall white matter changes. No significant association was observed between BRSD subscales and cognitive tests.
 |
Association of Regional White Matter Changes With Neuropsychiatric Symptoms
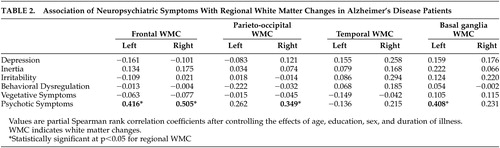
Table 2 summarizes partial Spearman correlation coefficients between regional white matter changes and neuropsychiatric symptom subscales in BRSD. The scores of the psychotic symptoms subscale were significantly associated with white matter changes in both frontal, right parieto-occipital, and left basal ganglia region. No significant correlation was noted between any regional white matter changes and other BRSD subscales, except psychotic symptoms.
 |
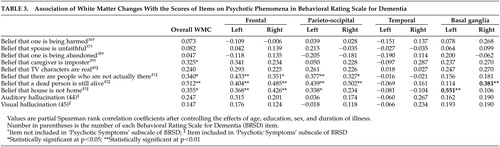
In order to clarify which specific types of psychotic symptoms were correlated with white matter changes, we further analyzed the relationships between each individual item included in the psychotic symptoms subscale (items 40–45) and white matter changes. We also performed the same kind of analyses for other BRSD items on psychosis (items 36–39), not included in the psychotic symptoms subscale, and white matter changes. The results from these analyses are summarized in Table 3 : belief that caregiver is imposter (Capgras syndrome) (item 39); belief that there are people who are not actually there (classical phantom boarder symptom) (item 41); belief that a dead person is still alive (item 42); and belief that house is not home (place misidentification) (item 43) were significantly correlated with overall white matter changes. Item 39 also significantly correlated with left frontal white matter changes. Item 41 significantly correlated with bilateral frontal and parieto-occipital white matter changes, and item 42 significantly correlated with bilateral frontal, bilateral parieto-occipital, and right basal ganglia. Item 43 significantly correlated with bilateral frontal, left parieto-occipital and left basal ganglia. Psychotic phenomenon-related items other than 39, 41, 42, and 43 were not associated significantly with overall or any regional white matter changes.
 |
DISCUSSION
This study showed that the severity of white matter changes significantly correlated with specific psychotic symptoms. As far as we know, this is the first report that demonstrates the relationship between white matter changes without infarcts or lacunae and psychotic phenomenon in Alzheimer’s disease patients.
The reason that previous studies conducted for Alzheimer’s disease subjects could not find the relationship between psychosis and white matter changes may be related to the nature of psychotic symptoms included in analyses. While there is no formal classification yet, the psychotic symptoms associated with Alzheimer’s disease could be divided into three groups: paranoid delusions, delusional misidentification, and hallucinations. 32 The psychotic symptoms subscale of BRSD, which significantly correlated with overall and regional white matter changes in our analyses, does not include items on paranoid delusion, but only includes delusional misidentification items, 32 , 33 (e.g., phantom boarder; belief that a dead person is still alive; belief that house is not home; capgras syndrome), and hallucination items (e.g., auditory and visual hallucination). In additional analyses on individual BRSD items for specific psychotic symptoms, only delusional misidentification items were significantly correlated with white matter changes; paranoid delusion and hallucination items were not. In contrast to our study, previous studies either did not assess or did not analyze delusional misidentification separately from paranoid delusion or hallucination. 6 – 11 Then, in case of hallucination, the number of patients with hallucinations (three patients with visual hallucination and three patients with auditory hallucination) was too small to draw a conclusion on the relationship with white matter changes.
Very limited numbers of studies 33 , 34 focused specifically on delusional misidentification associated with regional brain dysfunction in Alzheimer’s disease patients. Mentis et al., 34 referring to cerebral metabolic findings, suggested that abnormal frontal integration of perceptual information from multimodal association cortex and affective information from paralimic-limbic structures give rise to delusional misidentification in Alzheimer’s disease patients. Similarly, white matter changes in frontal or parieto-occipital region may possibly disrupt the functional connection between frontal cortex and other association cortex or paralimbic-limbic structures and, as a result, lead to delusional misidentification. White matter changes of basal ganglia also may contribute to the development of delusional misidentification through frontal-subcortical connection. 35
A CT study conducted by Förstl et al. 33 also indicated an accentuated degeneration of the right frontal lobe with relative preservation of the left frontal lobe in association with delusional misidentification. In our study, the correlation coefficients between white matter changes and delusional misidentification symptoms were greater relatively for the right side than for the left side. These findings support the importance of right side cerebral dysfunction for the development of delusional misidentification phenomena.
Depression, 6 apathy, 7 suicidal ideation, 8 and aberrant motor behaviors 9 associated with white matter changes in Alzheimer’s disease patients have been reported by some authors. However, none of these findings was replicated by other studies. We also could not find any significant relationship between such symptoms and white matter changes. The inconsistent findings may arise from differences in study subjects, and definition and quantification method of white matter changes as well as in neuropsychiatric symptom assessment. One study included not only Alzheimer’s disease patients, but dementia patients with other causes. 6 Another selected only Alzheimer’s disease patients with no vascular risk factors. 8 Excluding the cases with vascular risk factors may result in excluding severe white matter changes and distorting the clinical significance of white matter changes. In contrast, we targeted probable Alzheimer’s disease patients without any evidence of definite cerebrovascular disease, but did not exclude cases with vascular risk factors, unless they were serious enough to alter present mental function. In some studies, periventricular changes in the form of caps or smooth halos were included in the white matter changes category, 9 , 10 but not in other studies including ours. Caps or smooth halos are known to be of nonischemic origin, while other white matter changes represent ischemic tissue damage and have different clinical significance compared to halos or caps. 11 , 36 , 37
Some possible limitations should be discussed. First, we did not consider the degree of cortical brain atrophy as a covariate in correlation analyses because there was little evidence supporting the significant relationship between regional cortical atrophy and neuropsychiatric symptoms in Alzheimer’s disease. Some functional imaging studies using single photon emission tomography or positron emission tomography, however, indicated that regional cortical dysfunctions were associated with certain neuropsychiatric manifestations including psychotic symptoms. 34 , 38 – 40 Further multimodal imaging studies are, therefore, needed to differentiate regional cortical and subcortical contributions to the development of neuropsychiatric symptoms in Alzheimer’s disease. Second, as described in the methods section, we did not make any adjustment of a significance level for multiple statistical comparisons because this study was an exploratory one. While we can get very important clues about the relationships between white matter changes and neuropsychiatric manifestations in Alzheimer’s disease, the possibility of false positive associations cannot be excluded. Therefore, the findings from this study need to be replicated before they can be confirmed.
In conclusion, the results of this study indicate that white matter changes in Alzheimer’s disease patients, especially located in frontal and parieto-occipital regions, probably contribute to the development of a specific type of psychotic symptom, namely, delusional misidentification. Other neuropsychiatric or behavioral symptoms seem not to be related with white matter changes.
1. Roman GC: Senile dementia of the Binswanger type: a vascular form of dementia in the elderly. J Am Med Assoc 1987; 258:1782–1788Google Scholar
2. Caplan LR: Binswanger’s disease—revisited. Neurology 1995; 45:626–633Google Scholar
3. Scheltens P, Barkhof F, Valk J, et al: White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease: evidence for heterogeneity. Brain 1992; 115:735–748Google Scholar
4. Scheltens P, Barkhof F, Leys D, et al: Histopathological correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 1995; 45:883–888Google Scholar
5. Fazekas F, Kapeller P, Schmidt R, et al: The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer’s disease. J Neurol Sci 1996; 142:121–125Google Scholar
6. O’Brien J, Perry R, Barber R, et al: The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann N Y Acad Sci 2000; 203:482–489Google Scholar
7. Starkstein SE, Sabe L, Vazquez S, et al: Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1997; 63:66–73Google Scholar
8. Lopez OL, Becker JT, Reynolds CF III, et al: Psychiatric correlates of MR deep white matter lesions in probable Alzheimer’s disease. J Neuropschiatry Clin Neurosci 1997; 9:246–250Google Scholar
9. Hirono N, Kitagaki H, Kazui H, et al: Impact of white matter changes on clinical manifestation of Alzheimer’s disease: a quantitative study. Stroke 2000; 31:2182–2188Google Scholar
10. Harrell LE, Duvall E, Folks DG, et al: The relationship of high-intensity signals on magnetic resonance images to cognitive and psychiatric state in Alzheimer’s disease. Arch Neurol 1991; 48:1136–1140Google Scholar
11. Lopez OL, Becker JT, Rezek D, et al: Neuropsychiatric correlates of cerebral white-matter radiolucencies in probable Alzheimer’s disease. Arch Neurol 1992; 49:828–834Google Scholar
12. Binetti G, Padovani A, Magni E, et al: Delusions and dementia: clinical and CT correlates. Acta Neurol Scand 1995; 91:271–275Google Scholar
13. Filley CM, Gross KF: Psychosis with cerebral white matter disease. Neuropsychiatry Neuropsychol Behav Neurol 1992; 5:119–125Google Scholar
14. Hyde TM, Ziegler JC, Weinberger DR: Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol 1992; 49:401–406Google Scholar
15. Grant I: Neuropsychological and psychiatric disturbance in multiple sclerosis, in Multiple Sclerosis. Edited by McDonald WI, Silberberg. London, Butterworths, 1986, pp 134–152Google Scholar
16. Lawrence RM, Hillam JC: Psychiatric symptomatology in early-onset Binswanger’s disease: two case reports. Behav Neurol 1995; 8:43–46Google Scholar
17. Rice E, Gendelman S: Psychiatric aspects of normal pressure hydrocephalus. J Am Med Assoc 1973; 223:409–412Google Scholar
18. Lying-Tunnell U: Psychotic symptoms in normal-pressure hydrocephalus. Acta Psychiatr Scand 1979; 59:415–419Google Scholar
19. Lee JH, Lee KU, Lee DY, et al: Development of the Korean version of the consortium to establish a registry for Alzheimer’s disease assessment packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol Psychol Sci 2002; 57:47–53Google Scholar
20. Morris JC, Heyman A, Mohs RC, et al: The consortium to establish a registry for Alzheimer’s disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–1165Google Scholar
21. Hughes CP, Berg L, Danziger WL, et al: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Google Scholar
22. Rosen WG, Terry RD, Fuld P, et al: Pathological verification of ischemic score in differentiation of dementia. Ann Neurol 1979; 7:486–488Google Scholar
23. American Psychiatric Association: Diagnostic and statistical manual of mental disorders, 4 th ed. Washington, DC, American Psychiatric Association, 1994 Google Scholar
24. McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 1984; 34:939–944Google Scholar
25. Tariot PN, Mack JL, Patterson MB, et al: The CERAD behavioral rating scale for dementia (BRSD). Am J Psychiatry 1995; 152:1349–1357Google Scholar
26. Patterson MB, Mack JL: CERAD behavior rating scale for dementia (BRSD). Alzheimer Dis Assoc Disord 1997; 11(suppl 2):S90–S91Google Scholar
27. Wahlund LO, Barkhof F, Fazekas F, et al: A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32:1318–1322Google Scholar
28. Burns A, Jacoby R, Levy R: Psychiatric phenomena in Alzheimer’s disease, I: disorders of thought contents. Br J Psychiatry 1990; 157:72–76Google Scholar
29. Ballard C, Bannister C, Graham C, et al: Associations of psychotic symptoms in dementia sufferers. Br J Psychiatry 1995; 167:537–540Google Scholar
30. Welsh K, Butters N, Mohs RC: The consortium to establish a registry for Alzheimer’s disease (CERAD), part V: a normative study of the neuropsychological battery. Neurology 1994; 44:609–614Google Scholar
31. Almkvist O, Wahlund L-O, Andersson-Lundman G, et al: White-matter hyperintensity and neuropsychological functions in dementia and healthy aging. Arch Neurol 1992; 49:626–632Google Scholar
32. Rubin EH, Drevets WC, Burke WJ: The nature of psychotic symptoms in senile dementia of the Alzheimer type. J Geriatr Psychiatry Neurol 1988; 1:16–20Google Scholar
33. Förstl H, Burns A, Jacoby R, et al: Neuroanatomical correlates of clinical misidentification and misperception in senile dementia of the Alzheimer’s type. J Clin Psychiatry 1991; 52:268–271Google Scholar
34. Mentis MJ, Weinstein EA, Horwitz B, et al: Abnormal brain glucose metabolism in the delusional misidentification syndromes: A positron emission tomography study in Alzheimer’s disease. Biol Psychiatry 1995; 38:438–449Google Scholar
35. Cummings JL, Mega MS: Neuropsychiatry and behavioral neuroscience. New York, Oxford University Press, 2003, pp 7–23Google Scholar
36. Leifer D, Buonanno FS, Richardson EP, Jr: Clinicopatholgic correlations of cranial magnetic resonance imaging of periventricular white matter. Neurology 1990; 40:911–918Google Scholar
37. Fazekas F, Kleinert R, Offenbacher H, et al: Pathological correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43:1683–1689Google Scholar
38. Mega MS, Lee L, Dinov ID, et al: Cerebral correlates of psychotic symptoms in Alzhiemer’s disease. J Neurol Neurosurg Psychiatry 2000; 69:167–171Google Scholar
39. Hirono N, Mori E, Ishii K, et al: Frontal lobe hypometabolism and depression in Alzheimer’s disease. Neurology 1998; 50:380–383Google Scholar
40. Craig AH, Cummings JL, Fairbanks L, et al: Cerebral blood flow correlates of apathy in Alzheimer’s disease. Arch Neurol 1996; 53:1116–1120Google Scholar



