Variation in Neurophysiological Function and Evidence of Quantitative Electroencephalogram Discordance: Predicting Cocaine-Dependent Treatment Attrition
There are a number of factors that could make it difficult to detect treatment efficacy in randomized clinical trials. First, the cocaine-dependent population may be heterogeneous, making it difficult to detect beneficial effects of medication. 18 For example, in studies of depression, some investigators have reported that only participants with higher levels of depression are likely to benefit from both tricyclic antidepressants 4 and buproprion. 3 Second, high study attrition rates from medication treatment trials may make it difficult to detect treatment efficacy. Many participants discontinue their participation in trials (commonly to return to cocaine use), while other participants are terminated from trials by investigators because of failure to follow protocols (e.g., not taking study medication) or other problems (e.g., incarceration). Smelson et al. 19 demonstrated this typical problem with a 50% attrition rate, limiting the validity of their study results. In a study by Shaner et al., 20 50% of stimulant users with positive urine screen denied use. Clinical heterogeneity, subject attrition, or other factors may render a study insufficient to prove clear superiority of medication over placebo. 4 , 18
No symptom complexes or other clinical factors have been reported to be useful in consistently identifying subgroups of cocaine-dependent participants prior to a cocaine treatment trial: both exposure to and withdrawal from stimulants can alter neurochemistry and physiology. 21 , 22 However, the use of physiological measures to identify homogeneous groups of participants shows promise for enhancing the success of treatment trials for cocaine dependence. Kampman et al. 23 were able to predict attrition but not completion with urine toxicology screens upon admission. By adding a second measure which assessed withdrawal symptoms, the Cocaine Selective Severity Assessment (CSSA), accurate identification of participants’ outcomes increased significantly. 24 A weakness of both studies was a failure to monitor urine collection directly.
Previous work suggests that quantitative electroencephalography (QEEG) may be a useful physiologic measure for characterizing brain function in cocaine-dependent participants and could eliminate potential validity issues surrounding urine collection. There are significant shifts in QEEG power in cocaine-dependent participants, 25 with a significant association between power and the amount of cocaine used in the preceding week. 26 However, previous electroencephalogram (EEG) studies have not demonstrated an association between QEEG power measurements and treatment outcome. In this study, we examined cocaine-dependent participants using QEEG cordance, a measure that has moderately strong associations with cerebral perfusion and metabolism measured with positron emission tomography (PET) or single photon emission computed tomography (SPECT). 27 , 28 We examined participants at the beginning of a medication trial to determine if pretreatment measurements of brain function could be used to predict subject retention or other outcomes of treatment.
METHOD
Subjects
Twenty-five participants between the ages of 20 and 52 (the male to female ratio was four to one, mean = 35 [SD = 7]) meeting DSM–III–R criteria for cocaine dependence were recruited from the inpatient and outpatient programs of the West Los Angeles VA Medical Center, as well as from the West Los Angeles community. All participants were required to have a history of at least 3 months of cocaine dependence, self-reported use of $50 or more per week for the previous 4 weeks, and a positive urine screen within the 30 days preceding treatment. Prior to participation, each subject was required to sign a consent form after the procedures had been fully explained. Exclusionary criteria included concurrent dependence on any other psychoactive substance except nicotine, caffeine, or alcohol; any concomitant DSM–III – R axis I diagnosis; Beck Depression Inventory (BDI) score≥24; or presence of any significant active medical or neurological disease (particularly cardiovascular disease or hypertension). Any participants taking other medications that could interfere with the trial were also excluded, as were women of childbearing age not practicing medically-accepted birth control.
Screening and Evaluation Procedures
Participants meeting the above criteria underwent a complete medical history, physical examination, psychological evaluation, blood chemistry panel (Chem 25), complete blood count (CBC) and differential, urine drug screening, and for women, a serum pregnancy test. After this second evaluation, participants still meeting entry criteria were enrolled in a week-long baseline compliance evaluation that included a single-blind placebo wash-in and three clinic visits. During this week, we confirmed diagnosis with the Structured Clinical Interview for DSM–III–R, and evaluated participants using a brief clinical mental status examination, the Beck Depression Inventory (BDI), the Hamilton Depression Rating Scale (HAM-D), and the Addiction Severity Index (ASI). We also performed urine tests for benzoylecgonine, cannabinoids, amphetamines, opioids and benzodiazepines.
QEEG Procedure
Participants received a quantitative EEG (QEEG), performed by a registered technologist using a 19-channel referential montage based upon the International 10–20 System and applied according to standard clinical procedure. Recordings were performed in a sound-attenuated room, while participants rested in the eyes-closed, maximally alert state. 29 The signal from each EEG channel was digitized at 128 samples/sec/channel, with a high-frequency filter of 35 Hz and a low-frequency filter of 0.3 Hz. A technologist reviewed each recording and selected the first 20–32 seconds of artifact-free data for processing. I (the first author) then reviewed these selections to confirm the absence of artifact in the chosen data and processed them by fast Fourier transform to obtain absolute and relative power values for four Hz bands (0.5–4, 4–8, 8–12, and 12–20Hz) using the QND system (Neurodata, Inc., Pasadena, CA). A reattribution montage 30 was employed that provides good correlation between QEEG power values and cortical perfusion. The resulting montage is similar to the source derivation described by Hjorth, 31 but differs in that averaging is performed on power values after transformation to the frequency domain, as opposed to averaging being performed on signal voltages in the time domain.
Cordance Calculations
I calculated cordance values for each recording site in the four frequency bands using the algorithm summarized below. 28 Cordance has been shown to have stronger correlation with perfusion values than with either absolute or relative power values used independently. 28 Cordance is also more strongly associated with SPECT and PET than power alone. 32
A three-step algorithm 28 was employed to derive cordance values for each of the sites recorded. In the first step, absolute power was calculated for all the bipolar pairs of nearest-neighbor electrodes and reattributed from the electrode pairs to individual electrodes. This reattribution was accomplished by averaging EEG power for all pairs that included that electrode. 30 The averaged values were then square-root transformed to minimize both kurtosis and skewness. 33 Relative power was then calculated by dividing the absolute power in each frequency band for the total power for the entire spectrum.
The second step consisted of performing spatial normalization of both absolute and relative power across brain areas. This process involved averaging across all electrode sites in each frequency band f to derive mean absolute power and mean relative power value for the band. Z-scores were then calculated for each electrode site s in each frequency band. This resulted in normalized absolute and relative power values for each individual electrode site [Anorm(s,f) and Rnorm(s,f), respectively] with values expressed in z-score (SD) units.
The third step characterized electrodes by the association between Anorm (s,f) and Rnorm(s,f), in comparison with their means. Each individual electrode in each frequency band could then be examined in terms of the relationship between absolute and relative power. If both absolute and relative power were above or below the mean value for that electrode, the relationship was positively associated and the electrode was identified as “concordant.” If either absolute or relative power was above the mean value for that individual electrode while the other was below its mean, the association was negative and the electrode was identified as “discordant.”
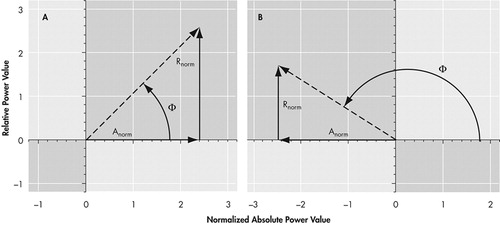
Because it is often useful to examine a global summary of an individual participant’s brain activity, a global brain state was then calculated in a fourth step. Using the cordance value calculated for each electrode site, the total proportion of electrodes displaying discordance for each individual is calculated. Two-thirds of normal control participants, as well as most depressed participants we examined, have fewer than 30% of electrodes in the discordant condition. 34 Based upon an examination of the frequency distribution of the proportion of discordant electrodes in the normal and depressed populations, we can divide study participants empirically into two categories that characterize the predominant global brain state: concordant and discordant. An individual may be designated with the discordant global characterization in two ways: by having a large number of electrodes showing discordance, or by having a few electrodes that are highly discordant (far from the mean values). Participants with a percentage of discordant electrodes of 30% or more are termed to exhibit the “discordant” state; those with a percentage of less than 30% are generally characterized as “concordant.” However, if a participant has two or more discordant electrodes that are highly deviant from the mean values (i.e., |Anorm| + | Rnorm| > 2.36 z-score units, or “extremely discordant”), then that subject is also termed to exhibit the discordant state. Data from simultaneous QEEG and PET studies 28 revealed that the concordance/discordance category for each electrode is related to perfusion: brain regions with concordance (electrodes in the upper right quadrant) showed the highest perfusion values ( Figure 1a ), while discordant brain regions (electrodes in the upper left quadrant) showed lower perfusion values (in Figure 1b ). In this project we confined our examination to cordance measures in the theta band for two reasons: our pilot data indicated that changes in brain electrical activity in this frequency band were most prominent among cocaine dependent participants, and theta band EEG activity has been associated with response outcomes in depression. 34 , 35
Experimental Procedure
All potential participants signed an informed consent form after experimental procedures were explained. Participants who then successfully completed a week-long compliance baseline underwent a random double-blinded assignment to receive either daily selegiline (10 mg) or a matching placebo for 8 weeks. All participants were required to complete three visits per week during which they took their medications under observation, provided urine samples for drug monitoring, and completed rating scales. The participants received one hour of individual counseling and three sessions of group therapy per week. We examined clinical ratings collected during the baseline compliance assessment and Week 1 of the trial as possible predictors of study retention. QEEG data were collected during the first 10 days of the study from all but three participants who were not available. One of these participants was recorded in Week 2 and two were recorded in Week 3 of the study. All three were characterized with the discordant brain state, but were evenly divided among the three outcome groups, so their data were included in the analyses. We examined retention data from the entire 8 weeks of the study to chart successful completion or early departure from the trial.
Data Analysis
We examined variables of age, depression level, dependence, and QEEG power collected during the baseline compliance week and Week 1 of the study using analysis of variance (ANOVA) and analysis of covariance (ANCOVA) to determine usefulness in predicting subject retention. Retention was defined as a participant remaining in the trial for the entire 9 weeks (1 week of baseline compliance and 8 weeks of drug trial). Dropouts were defined as participants who finished the baseline compliance week, but voluntarily withdrew while still meeting eligibility requirements. Dismissed participants were those who were removed involuntarily from the study after baseline for activities that violated the protocol (such as missing visits). We also examined cordance employing a Kruskal-Wallis ANOVA with a Mann-Whitney U-Wilcoxon Rank Sum W Test post hoc analysis to elucidate the ability of cordance to predict subject retention.
RESULTS
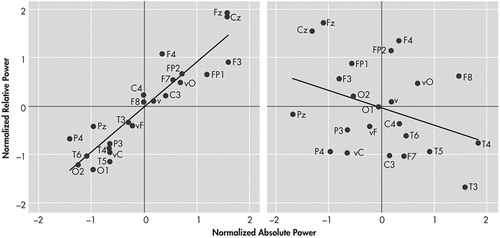
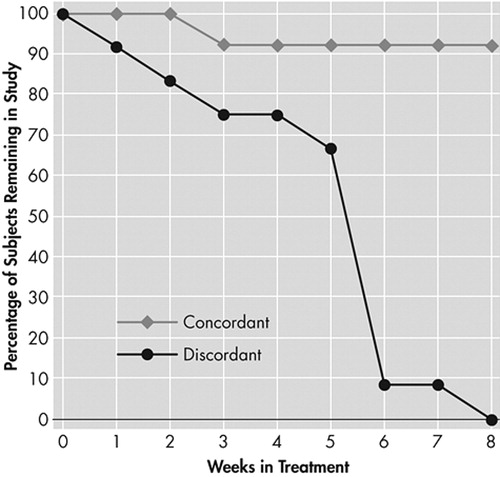
Of the 25 participants examined, 14 successfully completed the study, five dropped out and six were removed. There was a statistically significant difference in age (F=3.54 (2, 24) p=0.04) which was accounted for by the difference between those participants who remained in the study and those who were dismissed (Tukey-B p<0.05). Three of the five women enrolled in the study remained, while 11 of the 20 men enrolled remained ( Table 1 ). There was only a weak association between some of the clinical factors and attrition from the study. The BDI, Ham-D scores, and urine levels of benzoylecgonine from the baseline compliance week were not significant predictors of completion. There was a statistical trend toward a difference in urine levels of benzoylecgonine between participants who completed and those who dropped out of the study. Voluntary reports of “wanting” (F=3.26 (2, 24) p=0.06) and “craving” (F=2.62 (2, 24) p=0.10) cocaine were higher in those participants who were dismissed from the study, but were not significant predictors of retention after urine levels of benzoylecgonine were controlled for by ANCOVA. There was a difference among the groups in BDI scores at Week 1 (F=8.20 (2, 21) p=0.003), attributable to a difference between those who were dismissed and the other two groups employing a Tukey-B post hoc (p<0.05). The difference remained significant after controlling for the effects of age and benzoylecgonine by ANCOVA with variables entered simultaneously (F=5.48 (2, 21) p=0.02). Neither absolute nor relative QEEG power differed among those who completed and those who did not complete the medication trial when examined by ANOVA. Cordance measures did, however, detect a difference between those who completed and those who did not complete the study. A Kruskal-Wallis ANOVA showed that discordant participants were significantly less likely to complete the treatment trial than participants exhibiting the concordant global brain state (Chi-Square Likelihood Ratio = 13.34 (2), p=0.006). Figure 2 left illustrates a pattern of cordance, and right, a pattern of discordance. There was a significant difference between those who completed, those who dropped out, and those who were dismissed by a Mann-Whitney-Wilcoxon post hoc test (Z=−2.26, p=0.02) and (Z=−2.76, p=0.006), respectively ( Figure 3 ). The rate of attrition according to the baseline level of cordance is shown in Figure 4 . Concordant participants exhibited 92% retention through Week 2 of the study, and a final attrition rate of only 15%, as opposed to 83% and 100%, respectively, for discordant participants.
 |

DISCUSSION
These results suggest that there is variation in the neurophysiological function of potential cocaine-dependent study participants prior to enrollment in a medication treatment trial for dependency. In addition, this variation may be related to the probability of trial completion, dismissal of or dropout by the participant. A significant percentage of participants who successfully completed the trial were classified as concordant in their brain state as opposed to those who did not complete the trial. The neurophysiological differences reported here are significant even after controlling for benzoylecgonine levels, suggesting that there are fundamental differences in brain function which are independent of recent drug use. The differences in outcome among these participants cannot be explained on the basis of the many common clinical factors that contribute to heterogeneity in clinical trials. 36 Consistent with previous research, the levels of depression or craving 23 were not powerful factors in explaining participant retention. Those who were removed from the study did have slightly higher levels of depression than either those who remained or dropped out, but there was considerable overlap in the range of depression scores among the three outcome groups. Congruent with Kampman et al.’s work, 24 we observed a statistical trend toward a difference in levels of benzoylecgonine between those who completed and those who dropped out; however, this indicator did not reach the level of significance. Because of the moderately strong association between cordance and cerebral perfusion, 28 one may hypothesize that the participants who completed the study had higher levels of cerebral perfusion globally, and therefore may have suffered less brain dysfunction than those participants who dropped out or were dismissed. Studies of cerebral perfusion in cocaine-dependent participants without apparent neurological defects have shown widespread perfusion defects. 37 It is important to note, however, that no direct measurements of perfusion were performed on any of the participants in this study. Future studies of treatment retention should examine other indicators of brain dysfunction in cocaine dependent trial participants, including brain structure using magnetic resonance imaging (MRI) as well as neuropsychological test results. For example, cocaine dependence has been associated with an arrest of normal white matter volume increase as well as evidence of premature white matter damage with age. 18 , 38 The participants that were dismissed were significantly older, more dysfunctional (as seen in their inability to follow protocol), and more depressed. Further examination with measures such as single-photon emission computed tomography (SPECT) or functional magnetic resonance imaging (fMRI) could elucidate specific brain structures giving rise to concordant and discordant brain states. No conclusions can be drawn about the relationship between baseline cordance characteristics and effectiveness of the medication examined in the treatment trial. This study used only retention, a crude indicator of treatment outcome, as the measure of interest. The small sample size precludes our examining indices of treatment effectiveness, and the medications were assigned randomly and not according to the neurophysiological characteristics of the participants. Internal validity would have been increased if participant baseline QEEG measures were all obtained at identical abstinence length. Our inability to collect baseline measures at identical times may have confounded our measure, with differences in brain activity dependent upon length of abstinence or initiation of treatment. Future studies with more participants should examine the relationship between neurophysiological measures and clinical indicators of outcome such as frequency and/or intensity of use, periods of abstinence, functional impairment, or derived measures such as the treatment effectiveness score (TES). 39 Examination of these types of outcome measures will help determine whether neurophysiological heterogeneity is related to the overall functional outcome in cocaine-dependent participants.
1. Feingold A, Oliveto A, Schottenfeld R, et al: Utility of crossover designs in clinical trials: efficacy of desipramine vs placebo in opioid-dependent cocaine abusers. Am J Addict 2002; 11:111–123Google Scholar
2. Batki SL, Washburn AM, Delucchi K, et al: A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend 1996; 41:137–142Google Scholar
3. Margolin A, Kosten TR, Avants SK, et al: A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend 1995; 40:125–131Google Scholar
4. Nunes EV, McGrath PJ, Quitkin FM, et al: Imipramine treatment of cocaine abuse: possible boundaries of efficacy. Drug Alcohol Depend 1995; 39:185–195Google Scholar
5. Brady KT, Sonne SC, Malcolm RJ, et al: Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol 2002; 10:276–285Google Scholar
6. Gawin FH, Kleber HD, Byck R, et al: Desipramine facilitation of initial cocaine abstinence. Arch Gen Psychiatry 1989; 46:117–121Google Scholar
7. Crosby RD, Pearson VL, Eller C, et al: Phenytoin in the treatment of cocaine abuse: a double-blind study. Clin Pharmacol Ther 1996; 59:458–468Google Scholar
8. Handelsman L, Rosenblum A, Palij M, et al: Bromocriptine for cocaine dependence:a controlled clinical trial. Am J Addict 1997; 6:54–64Google Scholar
9. Levin FR, McDowell D, Evans SM, et al: Pergolide mesylate for cocaine abuse: a controlled preliminary trial. Am J Addict 1999; 8:120–127Google Scholar
10. Malcolm R, Kajdasz DK, Herron J, et al: A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend 2000; 60:161–168Google Scholar
11. Kolar AF, Brown BS, Weddington WW, et al: Treatment of cocaine dependence in methadone maintenance clients: a pilot study comparing the efficacy of desipramine and amantadine. Int J Addict 1992; 27:849–868Google Scholar
12. Schubiner H, Saules KK, Arfken CL, et al: Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol 2002; 10:286–294Google Scholar
13. Cornish JW, Maany I, Fudala PJ, et al: A randomized, double-blind, placebo-controlled study of ritanserin pharmacotherapy for cocaine dependence. Drug Alcohol Depend 2001; 61:183–189Google Scholar
14. Jenkins SW, Warfield NA, Blaine JD, et al: A pilot trial of gepirone vs placebo in the treatment of cocaine dependency. Psychopharmacol Bull 1992; 28:21–26Google Scholar
15. Rosse RB, Alim TN, Fay-McCarthy M, et al: Nimodipine pharmacotherapeutic adjuvant therapy for inpatient treatment of cocaine dependence. Clin Neuropharmacol 1994; 17:348–358Google Scholar
16. Oslin DW, Pettinati HM, Volpicelli JR, et al: The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat 1999; 16:163–167Google Scholar
17. de Lima MS, de Oliveira Soares BG, Reisser AA, et al: Pharmacological treatment of cocaine dependence: a systematic review. Addiction 2002; 97:931–949Google Scholar
18. Bartzokis G, Goldstein IB, Hance DB, et al: The incidence of T 2 -weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. Am J Neuroradiol 1999; 20:1628–1635 Google Scholar
19. Smelson DA, Losonczy MF, Davis CW, et al: Risperidone decreases craving and relapse in individuals with schizophrenia and cocaine dependence. Can J Psychiatry 2002; 47:671–675Google Scholar
20. Shaner A, Khalsa E, Roberts L, et al: Unrecognized cocaine use among schizophrenic patients. Am J Psychiatry 1993; 150(5):758–762Google Scholar
21. Nestler EJ: Cellular and molecular mechanisms of addiction, in Neurobiology of Mental Illness. Edited by Charney DS, Nestler EJ, Bunney BS. New York, Oxford University Press, 1999, pp. xix, 958Google Scholar
22. Newton TF, Cook IA, Kalechstein AD, et al: Qualitative EEG abnormalities in recently abstinent methamphetamine dependent individuals. Clin Neurophysiol 2003; 114:410–415Google Scholar
23. Kampman KM, Alterman AI, Volpicelli JR, et al: Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav 2001; 15:52–59Google Scholar
24. Kampman KM, Volpicelli JR, Mulvaney F, et al: Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav 2002; 27:251–260Google Scholar
25. Herning RI, Glover BJ, Koeppl B, et al: Cocaine-induced increases in EEG alpha and beta activity: Evidence for reduced cortical processing. Neuropsychopharmacology 1994; 11:1–9Google Scholar
26. Herning RI, King DE: EEG and evoked potentials alterations in cocaine-dependent individuals, in Neurotoxicity and Neuropathology Associated With Cocaine Abuse. National Institute on Drug Abuse Research Monograph Series, Monograph 163, Rockville, Md., National Institute on Drug Abuse, 1996Google Scholar
27. Leuchter AF, Cook IA, Dunkin JJ, et al: Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage 1994; 1:208–219Google Scholar
28. Leuchter AF, Uijtdehaage SHJ, Cook IA, et al: Relationship between brain electrical activity and cortical perfusion in normal subjects. Psychiatry Res 1999; 90:125–140Google Scholar
29. Leuchter AF, Dunkin J, Lufkin R, Anzai Y, et al: Effect of white-matter disease on functional connections in the aging brain. J Neurol Neurosurg Psychiatry 1994; 57:1347–1354Google Scholar
30. Cook IA, O’Hara R, Uijtdehaage SHJ, et al: Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol 1998; 107:408–414Google Scholar
31. Hjorth B: An online transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol 1975; 38:526–530Google Scholar
32. Leuchter AF, Cook IA, Mena I, et al: Assessing cerebral perfusion using quantitative EEG cordance. Psychiatry Research. Neuroimaging 1994; 55, 141–152Google Scholar
33. Leuchter AF, Daly KA, Rosenberg-Thompson S, et al: Prevalence and significance of electroencephalographic abnormalities in patients with suspected organic mental syndromes. J Am Geriatr Soc 1993; 41:605–611Google Scholar
34. Leuchter AF, Cook IA, Morgan ML, et al: Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry 2002; 159:122–129Google Scholar
35. Cook IA, Leuchter AF, Morgan M, et al: Early changes in prefrontal activity characterize clinical responders to antidepressants. Neuropsychopharmacology 2002; 27:120–131Google Scholar
36. Carroll KM, Nich C, Rounsaville BJ: Variability in treatment-seeking cocaine abusers: implications for clinical pharmacotherapy trials, in Medication Development for the Treatment of Cocaine Dependence: Issues in Clinical Efficacy Trials. Edited by B Tai, N Chiang, P Bridge. NIDA Research Monograph 175, U.S. Department of Health and Human Services, National Institutes of Health, 1997Google Scholar
37. Strickland TL, Mena I, Villanueva-Meyer J, et al: Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci 1993; 5:419–427Google Scholar
38. Bartzokis G, Beckson M, Lu PH, et al: Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry 2002; 51:605–612Google Scholar
39. Ling W, Shoptaw S, Wesson D, et al: Treatment effectiveness score as an outcome measure in clinical trials, in Medication Development for the Treatment of Cocaine Dependence: Issues in Clinical Efficacy Trials. Edited by B Tai, N Chiang, P Bridge. NIDA Research Monograph 175, U.S. Department of Health and Human Services, National Institutes of Health, 1997Google Scholar



