Abnormalities in Motor Physiology in Bipolar Disorder
We considered that if disturbances in basal ganglia functioning are important in bipolar disorder, then it is possible that patients with the condition might show abnormalities in aspects of motor physiology that have been considered to relate to the functioning of these structures. Over the years we have developed quantitative instrumental approaches to measuring abnormalities in motor function that appear to be related to basal ganglia dysfunction. 10 – 12 The two main methods we have developed assess the ability to maintain steady-state force and the ability to scale velocity with distance. Although we have observed that patients with clinically observable movement disorders show abnormalities on these measures (with abnormalities in force steadiness being seen in movement disorders marked by a hyperdopaminergic state, and velocity scaling abnormalities being associated more with a hypodopaminergic condition), 13 we also have found that patients without obvious clinical movement problems may demonstrate abnormalities on these measures. In particular, we have found that patients with schizophrenia may show abnormalities on these measures without evidence of frank tardive dyskinesia or parkinsonism. 14
We hypothesized that patients with bipolar disorder would show abnormalities on these measures of force steadiness and velocity scaling. Further, because of the possible importance of hyperdopaminergia in mania 15 , 16 and hypodopaminergia in depression, 17 , 18 we considered that performance on the force steadiness and velocity scaling measures would correlate with symptoms of mania and depression, respectively. In particular, we hypothesized that the degree of manic symptoms would positively correlate with force steadiness scores, and the degree of depressive symptoms would negatively correlate with velocity scaling scores (lower velocity scaling scores indicate less impairment). A third objective of the study was to explore whether impaired motor performance might relate to current pharmacotherapy.
METHOD
Subjects and Medication Status
We studied 67 individuals (39 men and 28 women) meeting DSM-IV criteria 19 for bipolar disorder and 47 healthy comparison subjects (31 men and 16 women). We recruited these subjects through advertisements placed in the local media and Internet sites for research recruitment. All subjects signed Institutional Review Board-approved consent for voluntary participation in this research. The mean age of the bipolar group was 44.3 (SD=10.9) years and the mean age for the healthy comparison group was 39.9 (SD=13.2) years, which was not significantly different. All patients had been either off medication or on stable doses of medication for at least 1 month prior to undergoing motor assessment. The breakdown of the bipolar disorder patients by medication type was as follows: unmedicated (N=11); mood stabilizers (N=46); antidepressants (N=37); antipsychotics (N=15); and benzodiazepines (N=10). Thirty-nine patients were taking two or more medication types at the time of testing.
Patients were excluded if there was any clinical evidence of neuroleptic-induced side effects such as parkinsonism or tardive dyskinesia, or if there was clinical evidence of tremor related to mood stabilizers, such as lithium or valproate.
Clinical Assessment of Diagnosis and Psychopathology
All patients underwent a Structured Clinical Interview for DSM-IV (SCID) performed by trained personnel. Sixty-three patients met criteria for bipolar type I disorder, and four patients for bipolar type II disorder. Clinical assessments included the 28-item Hamilton Depression Rating Scale (HAM-D) 20 for the severity of depression, and the Young Mania Rating Scale (YMRS) 21 for the severity of mania.
Instrumental Assessment of Motor Function
Force Steadiness
The force steadiness procedure has been in use in our laboratory for over 10 years. 11 , 22 In this procedure, subjects sit facing a computer monitor with one of their hands positioned atop a platform equipped with the load cell (Sensotec, model 31/1426–02, range=1000 g). A load cell mounted onto the platform transduces force applied by flexing the index finger. A target line equivalent to 350 centiNewtons of force and the subject’s force signal are displayed on the monitor. The two waveforms move at a constant speed from left to right across the computer monitor to fill a 20-second window. At the end of each 20-second window, the screen is refreshed.
Subjects were instructed to match the target with their force signal by maintaining steady levels of finger flexion force. Three 20-second trials were obtained, each separated by a 5-second rest. The force signal was sampled at 100 samples/second by a laboratory computer and stored for subsequent analysis. Analysis involved identifying the segment with the greater range in force (determined by computing the force minima and maxima over the medial 80% of each segment) and calculating the waveform mean and standard deviation. Both hands were assessed, and data from the hand with the higher score were used for analysis.
Because many patients taking lithium develop a high frequency tremor 23 which can interfere with performance of the force steadiness task as well as the interpretation of results, we excluded patients with clinical evidence of tremor due to mood stabilizers, as well as low pass-filtered the force waveform to remove high frequency oscillations (>3.0 Hz). The coefficient of variation obtained by dividing the standard deviation of the force waveform by the mean force waveform served as the force steadiness score. Higher scores indicate greater error or instability. Force steadiness error is the direct result of irregular muscle contractions that produce changes in measurable force over time. The force steadiness data acquisition procedure, segmentation, and analysis have been shown to be highly reliable and valid. 24Figure 1 shows examples of force steadiness waveforms from a healthy comparison subject ( Figure 1a ) and a subject with force steadiness impairment ( Figure 1b ).
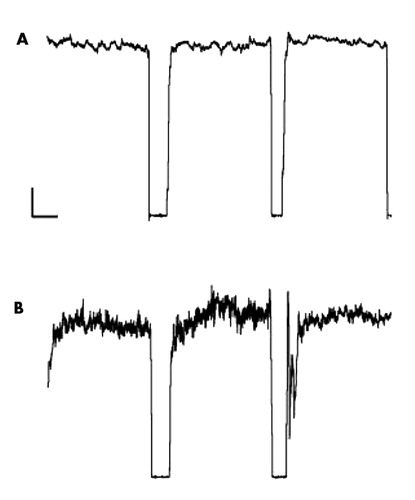
Shown are force tracings from three 20-second trials (with periods of rest in between) from a normal healthy subject (A) and a subject with force steadiness impairment (B). Force steadiness scores, calculated by dividing the standard deviation by the mean for second and third trials, were 1.6 for the healthy subject (A) and 8.4 for the impaired subject (B). Higher force steadiness scores indicate greater force instability. Calibration bar represents 50 cN and 5 seconds.
Velocity Scaling
The ability to scale movements is considered a cardinal function of the basal ganglia. 25 , 26 For example, some patients with parkinsonian hypokinesia execute all movements, regardless of distance or complexity, with a uniform velocity. 27 – 29 Investigators have shown that the slope of the relationship between movement velocity and target distance is sensitive to the extent of bradykinesia. 30 This phenomenon is consistent with recent studies showing that while Parkinson’s disease patients can increase movement velocity with increases in movement distance, 31 the slope of the relationship between peak velocity and target distance is often lower than in healthy comparison subjects. 32
For our measure of velocity scaling, we measured the velocity-distance relationship during simple wrist rotation using a platform equipped with a rotation sensor (electrogoniometer) that was attached to a handle. Rotation of the handle by flexing or extending the wrist produces a continuous signal that is proportional to the angle of rotation. We used custom software to convert the digitized signal (sampled at 100 samples/second) to a large cursor displayed on a computer monitor. The position of the cursor along the horizontal plane was calibrated in degrees of rotation. Along with the cursor, target boxes were displayed on the monitor along a horizontal plane located at 25° or 45°. Targets were placed left of midline for right wrist flexion, and right of midline for left wrist flexion. Subjects were instructed to flex the wrist “as quickly and as accurately as possible” when a target box appears on the screen. Subjects used visual feedback to place their cursor in the target box. The target remains were displayed for 2 seconds with an interstimulus interval of 2 seconds. If the subject needed clarification, instructions were repeated with emphasis on “speed of movement.” Thirty-two trials or movements, consisting of 16 trials for each of two randomly presented target locations, were administered for each hand, for a total of 64 movements. Velocity and distance difference scores were obtained by subtracting the respective values for the 25° targets from the 45° targets. The velocity scaling score was derived from the ratio of the velocity difference over the angular distance difference, scaled in degrees/second/degree. Lower scores indicate disturbances in the ability to scale movement velocity with distance. Both hands were assessed, and data from the hand with the lower score were used for analyses.
In previous studies we have determined that test-retest reliability for this measure is very high. 12 , 33Figure 2 shows velocity-displacement relationships for a single subject with normal ( Figure 2a ) and abnormal ( Figure 2b ) velocity scaling. In the healthy sample, there is a significant increase in velocity with increases in angle of wrist rotation. Such increases in velocity are not present in the case with abnormal velocity scaling.
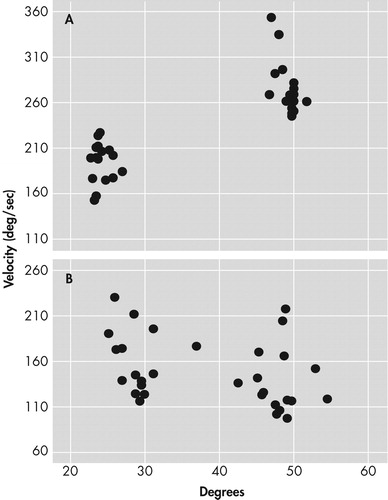
Figure A shows normal scaling represented by the increase in velocity with increasing angle of wrist rotation over 32 trials. Figure B shows a lack of scaling represented by the independent relationship between velocity and wrist rotation angle over 32 trials.
Motor Measure Characteristics
Eighteen psychiatrically and medically stable patients were retested 6 months following their initial assessment to examine measurement reliability. Correlational analyses indicated high levels of repeatability for both the force steadiness (r=0.86, p<0.001) and velocity scaling (r=0.75, p<0.001) measures.
Analyses of the relationships between the force steadiness and velocity scaling measures indicated that performance on one measure was independent of performance on the other. Pearson's product moment correlations were −0.03 for bipolar disorder and −0.17 for healthy comparison subjects. These findings support the specificity of our two motor measures.
There were no effects of gender or age on either the force steadiness or velocity scaling measures. Also, there were no differences on force steadiness or velocity scaling among the patients treated with different medications.
Statistical Methods
Group data were examined for homogeneity of variances and normal distributions prior to undertaking parametric statistical analyses. Group comparisons involving data meeting all assumptions for parametric analyses were subjected to analyses of variance (ANOVA) and post-hoc planned comparisons to test group differences. Separate analyses were performed for force steadiness and velocity scaling data. Group data that did not meet the assumptions for parametric analyses, even after transforming the raw scores, were subjected to Friedman’s ANOVA and post-hoc Mann-Whitney tests. Correlational analyses were used to examine relationships between motor scores and severity of depression and mania. Chi-square tests were used to examine differences in prevalence of motor abnormalities among patients who were on versus patients who were off medication.
Patients were also classified as “normal” or “abnormal” based on the healthy comparison subjects’ 95 th percentile score. Patients scoring above 2.26 on the force steadiness measure or below 1.88 on the velocity scaling measure were therefore classified as “abnormal.”
RESULTS
Overall, bipolar disorder patients performed significantly more poorly than healthy comparison subjects. The mean scores for the force steadiness and velocity scaling measures for the healthy comparison group were 1.96 (SD=0.77) and 2.53 (SD=1.35), respectively. The mean scores for the force steadiness and velocity scaling measures for the bipolar disorder patients were 3.46 (SD=3.00) and 1.71 (SD=0.92), respectively. Nonparametric tests for group differences revealed highly significant differences between bipolar disorder patients and healthy comparison subjects for the force steadiness (p<0.00001) and velocity scaling (p<0.001) measures.
For the force steadiness measure, 39 patients (58%) scored within the abnormal range. For the velocity scaling measure, 42 out of 67 patients (63%) scored within the abnormal range. Twenty-five bipolar disorder patients (37%) exhibited both force steadiness and velocity scaling abnormalities, and 56 patients (84%) had abnormal measures on either assessment. Scores on the force steadiness and velocity scaling measures were unrelated to each other. Also, the percentage of patients with abnormalities on both measures (37%) is no greater than would be calculated from a chance relationship between the two measures (58% x 63% = 36%).
Affective State
Patients with abnormal force steadiness scores did not differ from patients with normal force steadiness scores on ratings of depression or mania. Patients exhibiting both force steadiness and velocity scaling abnormalities did not differ from other subgroups on these ratings of mood pathology. There were no significant relationships between severity of mood symptoms based on the HAM-D and YMRS scores and the force steadiness or velocity scaling measures. The Pearson’s r values for the correlations between HAM-D and the motor measures were −0.19 (force steadiness) and −0.09 (velocity scaling), and values between YMRS and the motor measures were −0.06 (force steadiness) and 0.17 (velocity scaling).
Medication Effects
Because of the known influence of certain psychotropic medications on dyskinesia and parkinsonism, we examined whether the motor abnormalities described above might be related to current medication status. The bipolar disorder subjects were divided into those who were being treated with a particular class of psychotropic medications and those who were off these medications. Patients on current pharmacotherapy were separated into five groups, including subjects on antidepressants, mood stabilizers, antipsychotics, and benzodiazepines, and those off medication at the time of testing. We examined each medication group to determine if it was associated with a greater prevalence of instrumentally measured motor abnormalities. Prevalence results indicated no significant differences between the numbers of bipolar disorder subjects meeting criteria for force steadiness or velocity scaling impairment among any of the groups. Prevalence rates for medicated subjects ranged from 50% (for antipsychotics or benzodiazepines) to 62.5% (for antidepressants) for force steadiness impairment, and 50% (for benzodiazepines) to 80% (for antipsychotics) for velocity scaling impairment. Although there were only 11 unmedicated bipolar disorder subjects, seven of the 11 (63.6%) unmedicated bipolar disorder subjects exhibited abnormal force steadiness scores and five (53.5%) exhibited abnormal velocity scaling scores. Thus, the prevalence of instrumentally derived motor abnormalities among unmedicated bipolar disorder subjects was comparable to that of the medicated subjects.
DISCUSSION
We addressed three questions about motor pathophysiology in bipolar disorder. First, we examined whether bipolar disorder patients exhibit disturbances in motor physiology that may relate to abnormalities within the sub-cortical motor circuits. We found that approximately 60% of our bipolar disorder patients exhibited force steadiness or velocity scaling motor abnormalities based on instrumental assessments, with 84% of patients having abnormalities on either measure. Over one-third of the patients exhibited both forms of motor impairment. The second question of this study was to examine whether disturbances in motor physiology in bipolar disorder patients as assessed by force steadiness and velocity scaling were related to an affective state in bipolar disorder. We found no correlation between the motor scores and the severity of manic symptoms (as measured by the YMRS) or depressive symptoms (as measured by the HAM-D). The third question pertained to whether the observed impairment in motor functions was possibly related to current pharmacotherapy. We found no differences in prevalence of impairment between bipolar disorder patients on versus off psychotropic medications. Moreover, unmedicated bipolar disorder patients exhibited similar distributions of force steadiness and velocity scaling impairment as treated patients. The possibility remains that past exposure to certain medications could have contributed to the observed abnormalities in motor function.
The lack of associations between mood state, pharmacotherapy, and force steadiness or velocity scaling performance indicates that these motor impairments may be trait characteristics of bipolar disorder. However, caution is warranted in this interpretation because the present study was cross-sectional and not longitudinal in nature. Nevertheless, it is possible that these measures may be useful as endophenotypes in future genetic studies of bipolar disorder.
With the exception of psychomotor retardation 34 or lithium-induced tremor, 35 few studies have focused specifically on motor abnormalities in bipolar disorder. The few exceptions include studies by Heninger and Kirstein, 36 who reported increased motor activity in manic bipolar disorder patients, and Klein et al., 37 who reported that increased motor activity predicted relapse into mania in bipolar patients. It may be argued that this lack of attention to motor disturbances in bipolar disorder may be related to their relative infrequency in bipolar disorder in comparison to schizophrenia 38 , 39 or major depression. 34 Motor problems in bipolar disorder may also be underreported because they can be subtle and difficult to assess using conventional assessment means. 40
Our findings show that approximately 60% of patients with bipolar disorder demonstrate significant impairments in the ability to maintain steady-state force or the ability to scale velocity with distance; but these two findings appear to be independent, suggesting that two separate physiological mechanisms relating to basal ganglia circuitry may be involved in bipolar disorder. In fact, the putative neural circuits involved in motor dysfunction have been elucidated over the past two decades 41 , 42 and two basic pathways have been identified. Neurotransmission from the motor cortex, through the basal ganglia and thalamus, and back to the sensorimotor cortices has been proposed to follow a direct pathway and an indirect pathway, the latter of which involves the subthalamus. 43 Disturbances within the direct pathway have been suggested to be associated with the reduction of movement or with difficulty initiating movement (hypokinesia), whereas disturbances within the indirect pathway have been proposed to be associated with excessive or unstable movement (hyperkinesia). In this context, our results suggest that velocity scaling abnormalities may possibly relate to dysfunction in the direct pathway, and the force steadiness abnormalities to disturbances in the indirect pathway in bipolar disorder. Although this is highly speculative, these brain regions could serve as a focus in future studies utilizing techniques such as functional magnetic resonance imaging (fMRI).
Our findings suggest that psychopathology and motor dysfunction may share a common pathophysiology. Comorbid mood and motor disorder are common features in neuropsychiatric disorders, such as Parkinson’s and Huntington’s diseases. For example, depression is common among patients with Parkinson’s disease with prevalence estimates ranging from 20% to 45%. 43 , 44 Studies have shown that the onset of depressive symptoms often predates the presentation of motor signs in Parkinson’s disease. 45 This would imply that the mood symptoms may be more closely linked to the disease process than a secondary feature of Parkinson’s disease. Based on a study using positron emission tomography (PET), Remy et al. 46 suggested that depression in Parkinson’s disease might be associated with reduction in dopamine and norepinephrine receptor binding in structures including limbic regions.
Both depression and mania are observed in Huntington’s disease, a prototypic movement disorder of basal ganglia origin. Estimates of the prevalence of a mood disorder in Huntington’s disease range from 22% to 38% for depression 47 and 5% to 10% for mania. 48 PET studies in depressed Huntington’s disease subjects also show pathophysiological correlates (e.g., glucose hypometabolism in the orbitofrontal and inferior parietal regions of the brain) that are similar to those observed in major depression. 49
The co-occurrence of mood symptoms with parkinsonism and choreoathetosis in neurological diseases supports the hypothesis that hypokinetic or hyperkinetic motor abnormalities may coexist with affective disorders because of shared pathophysiology. The present findings of motor problems in bipolar disorder suggest an extension of this hypothesis to include psychiatric disorders as well. Although we did not observe a significant relationship between the severity of mania or depression and severity of motor dysfunction in this cross-sectional study, a longitudinal study might reveal that a fluctuation in affective state is related to a fluctuation in motor control.
In conclusion, the results from this study contribute to the literature by characterizing motor abnormalities in bipolar patients. The use of sensitive quantitative instrumentation of motor function may be helpful in characterizing the brain abnormalities of bipolar disorder. Moreover, better characterization of the motor circuits underlying these abnormalities may help us determine which circuits are disrupted in the modulation of affect and where to target novel treatments.
1. Blumberg HP, Stern E, Martinez D, et al: Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 2000; 48:1045–1052Google Scholar
2. Noga JT, Vladar K, Torrey EF: A volumetric MRI study of monozygotic twins discordant for bipolar disorder. Psychiatr Res Neuroimag Sect 2001; 106:25–34Google Scholar
3. Strakowski SM, Adler CM, DelBello MP: Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord 2002; 4:80–88Google Scholar
4. Blumberg HP, Martin A, Kaufman J, et al: Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 2003; 160:1345–1347Google Scholar
5. Caligiuri MP, Brown GG, Meloy MJ, et al: An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res 2003; 123:171–182Google Scholar
6. Shastry BS: Bipolar disorder: an update. Neurochem Int 2005; 46:273–279Google Scholar
7. Mukherjee S, Rosen AM, Caracci G, et al: Persistent tardive dyskinesia in bipolar patients. Arch Gen Psychiatry 1986; 43:342–346Google Scholar
8. Ries RK: DSM-III implications of the diagnosis of catatonia and bipolar disorder. Am J Psychiatry 1985; 142:1471–1474Google Scholar
9. Fein S, McGrath MG: Problems in diagnosing bipolar disorder in catatonic patients. J Clin Psychiatry 1990; 51:203–205Google Scholar
10. Caligiuri MP, Lohr JB: A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry 1994; 35:104–111Google Scholar
11. Caligiuri MP, Lohr JB: Instrumental motor predictors of neuroleptic-induced parkinsonism in newly medicated schizophrenia patients. J Neuropsychiatry Clin Neurosci 1997; 9:562–567Google Scholar
12. Caligiuri MP, Lohr JB, Ruck, RK: Scaling of movement velocity: a measure of neuromotor retardation in individuals with psychopathology. Psychophysiology 1998; 35:431–437Google Scholar
13. Caligiuri MP, Lohr JB: Worsening of postural tremor in patients with L-dopa-induced dyskinesia. Clin Neuropharmacol 1993; 16:244–250Google Scholar
14. Cortese L, Caligiuri MP, Malla AK, et al: Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res; 2005 75: 65–75.Google Scholar
15. Gerner RH, Post RM, Bunney WE Jr: A dopaminergic mechanism in mania. Am J Psychiatry 1976; 133:1177–1180Google Scholar
16. Yatham LN, Liddle PF, Shiah IS, et al: PET study of [(18)F]6-fluoro- l -dopa uptake in neuroleptic- and mood-stabilizer-naive first-episode nonpsychotic mania: effects of treatment with divalproex sodium. Am J Psychiatry 2002; 159:768–774 Google Scholar
17. Klimek V, Schenck J, Han H, et al: Dopaminergic abnormalities in amygdaloid nuclei in major depression: a post-mortem study. Biol Psychiatry 2000; 52:740–748Google Scholar
18. Laasonen-Balk T, Kuikka J, Viinameaki H, et al: Striatal dopamine transporter density in major depression. Psychopharmacology 1999; 144:282–285Google Scholar
19. APA: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington DC, American Psychiatric Association, 1994Google Scholar
20. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
21. Young RC, Biggs JT, Ziegler VE, et al: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Google Scholar
22. Caligiuri MP, Lohr JB: A potential mechanism underlying the voluntary suppression of tardive dyskinesia. J Psychiatr Res 1989; 23:257–266Google Scholar
23. Elble RJ, Koller WC: Tremor. Baltimore, Johns Hopkins University Press, 1990Google Scholar
24. Caligiuri MP, Lohr JB, Rotrosen J, et al: Reliability of an instrumental assessment of tardive dyskinesia: results from VA cooperative study #394. Psychopharmacology 1997; 32:61–66Google Scholar
25. Vaillancourt DE, Mayka MA, Thulborn KR, et al: Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage 2004; 23:175–186Google Scholar
26. Smiley-Oyen AL, Worringham CJ, Cross CL: Motor learning processes in a movement scaling task in olivopontocerebellar atrophy and Parkinson’s disease. Exp Brain Res 2003; 152:453–465.Google Scholar
27. Draper IT, Johns RJ: The disordered movement in parkinsonism and the effect of drug treatment. Bull Johns Hopkins Hosp 1964; 115:465–480Google Scholar
28. Berardelli A, Dick JPR, Rothwell JC, et al: Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1986; 49:1273–1279Google Scholar
29. Hufschmidt A, Lucking CH: Abnormalities of tracking behavior in Parkinson’s disease. Mov Disord 1995; 10:267–276Google Scholar
30. Warabi T, Noda H, Yanagissawa N, et al: Changes in sensorimotor function associated with degree of bradykinesia in Parkinson’s disease. Brain 1986; 109:1209–1224Google Scholar
31. Pfann KD, Penn RD, Shannon KM, et al: Pallidotomy and bradykinesia: Implications for basal ganglia function. Neurology 1998; 51:796–803Google Scholar
32. Pfann KD, Buchman AS, Comella CL, et al: Control of movement distance in Parkinson’s disease. Mov Disord 2001; 16:1048–1065Google Scholar
33. Caligiuri MP, Ellwanger J: Motor and cognitive aspects of motor retardation in depression. J Affect Disord 2000; 57:83–93Google Scholar
34. Sobin C, Sackeim HA: Psychomotor symptoms of depression. Am J Psychiatry 1997; 154:4–17Google Scholar
35. Gelenberg AJ, Jefferson JW: Lithium tremor. J Clin Psychiatry 1995; 56:283–287Google Scholar
36. Heninger GR, Kirstein L: Effects of lithium carbonate on motor activity in mania and depression. J Nerv Ment Disord 1977; 164:168–175Google Scholar
37. Klein E, Lavie P, Meiraz R et al: Increased motor activity and recurrent manic episodes: predictors of rapid relapse in remitted bipolar disorder patients after lithium discontinuation. Biol Psychiatry 1992; 31:279–284Google Scholar
38. Wolff A-L, O’Driscoll GA: Motor deficits and schizophrenia: the evidence from neuroleptic-naive patients and populations at risk. J Psychiatry Neurosci 1999; 24:304–314Google Scholar
39. Fenton WS: Prevalence of spontaneous dyskinesia in schizophrenia. J Clin Psychiatry 2000; 61(suppl 4):10–14Google Scholar
40. Sobin C, Mayer L, Endicott J: The motor agitation and retardation scale: a scale for the assessment of motor abnormalities in depressed patients. J Neuropsychiatry Clin Neurosci 1998; 10:85–92Google Scholar
41. Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 1986; 9:357–381Google Scholar
42. DeLong MR: Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13:281–285Google Scholar
43. Starkstein SE, Mayberg HS, Leiguardia R, et al: A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1992; 55:377–382Google Scholar
44. Tandberg E, Larsen JP, Aarsland D, et al: The occurrence of depression in Parkinson’s disease: a community-based study. Arch Neurol 1996; 53:175–179.Google Scholar
45. Richards H: Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J Neurol Neurosurg Psychiatry 2005; 76(suppl 1):48–52Google Scholar
46. Remy P, Doder M, Lees A, et al: Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005; 128:1314–1322Google Scholar
47. Folstein SE, Chase G, Wahl W, et al: Huntington’s disease in Maryland: clinical aspects of racial variation. Am J Hum Genet 1987; 41:168–179Google Scholar
48. Mendez MF: Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med 1994; 24:189–208Google Scholar
49. Mayberg HS, Starkstein SE, Peyser CE, et al: Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology 1992; 42:1791–1797Google Scholar



