Brain Response Correlates of Decisional Capacity in Schizophrenia
The few published studies that have used a comprehensive neuropsychological battery to examine support for this idea 5 , 9 , 11 , 12 have found evidence for a general relationship between cognition and capacity to consent, but less evidence for specificity. Palmer and Jeste 11 suggest that the use of clinical neuropsychological measures, which often rely on multiple information processing domains within a single test, may make it difficult to detect the relationship of particular domains with elements of consent-related capacity. It has been argued that functional neuroimaging is a more sensitive measure of individual differences among patients with schizophrenia than behavioral measures (e.g., in the realm of genetics). 13 Thus, functional imaging may be a better tool for examining sources of variability in decisional capacity than neuropsychological measures alone.
In this study, we measured decisional capacity using a well-validated instrument, the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). We focused on understanding and reasoning abilities because of the greater degree of measurement reliability and subject variability in these subscales compared with the appreciation and expression of choice subscales. 8 We postulated that two crucial neurocognitive systems underlie some of the observed deficits in understanding and reasoning among patients with schizophrenia. Understanding of consent-related information presumably requires both encoding and retrieval of information about study procedures. Episodic learning and retrieval deficits are prevalent among individuals with schizophrenia, 14 , 15 and functional neuroimaging studies have found inferior frontal and medial temporal brain abnormalities during encoding and retrieval among patients. 16 – 21 Reasoning is usually assessed by asking participants to make mental comparisons between potential consequences of participation (including possible adverse effects) and therefore, like a working memory task, would seem to require simultaneous manipulation of information. Working memory abilities are frequently disrupted in schizophrenia, 22 and dysfunction of the dorsolateral prefrontal cortex consistently has been implicated in these deficits. 23 – 25 Thus, we postulated that performance on measures related to the understanding of consent-related information would be associated with medial temporal and inferior frontal brain function, whereas reasoning would be related to brain response of the lateral prefrontal cortex (i.e., middle frontal gyrus). We chose to measure brain response during a verbal learning task because of its relevance to the consent process, and because it would likely challenge both medial temporal and frontal brain regions. We studied middle-aged and older patients because of the increased risk of cognitive deficits that may impair decisional capacity in this population. 26
We hypothesized that the functional magnetic resonance imaging (fMRI) response of patients with schizophrenia in the medial temporal and inferior prefrontal cortex would correlate positively with MacCAT-CR understanding scores, whereas lateral prefrontal brain response (i.e., in the middle frontal gyrus) would positively relate to reasoning scores.
METHOD
Participants
We recruited 24 community-dwelling outpatients with a DSM-IV-based 27 diagnosis of schizophrenia consecutively from Board-and-Care homes in San Diego county, Calif. Following an assessment of decisional capacity, five patients were deemed incapable of giving informed consent for the functional imaging study. Scanning was not completed in three additional patients, and data were not usable for another two patients due to excessive motion during the scan. Therefore, data were available for 14 schizophrenia patients.
Assessment of Decisional Capacity
The study was approved by the University of California, San Diego, Institutional Review Board. After presenting consent information for the fMRI study, “Understanding” and “Reasoning” were assessed using a version of the MacCAT-CR 28 tailored to the consent form for the present, nonhypothetical study. Participants were required to achieve a criterion score of at least 16 out of 26 correct 12 on the Understanding portion of the MacCAT-CR (the standard used in a large-scale national clinical trial) 29 in order to be considered capable of giving informed consent. If this was not achieved after one presentation of the consent form, up to two additional presentations of all consent information were made (differing from standard MacCAT-CR protocol, which calls for only one repetition of misunderstood information). Reasoning was assessed only after the first administration of the consent information, consistent with the recommendations of the MacCAT-CR manual. 28
Functional Imaging
Behavioral Task
Participants were presented with 32 pairs of associated nouns and instructed to learn “how they go together” for later testing. Half of the pairs were novel and half were familiar as they had been previously presented prior to scanning. Word-pair learning trials were presented in blocks of four trials each, interspersed with blocks of fixation of varying length. Each learning trial lasted for 5 seconds, and participants indicated with a button-press which of the words was capitalized, as a check that they were processing each stimulus. The following measures of performance were assessed: accuracy of indicating capitalization (during scanning) and post-scan cued-recall accuracy.
Scanning Procedure
Participants were scanned in a 1.5T Siemens Vision MRI scanner. Blood-oxygen-level-dependent (BOLD) response during the task was assessed with gradient-recalled echoplanar imaging (EPI) sequence (69 whole-brain images of 32 axial slices, 4 mm thickness, 4×4 mm in-plane resolution, TR=4000 msec; TE=40 msec; flip angle=90°). High-resolution anatomical images were acquired using the magnetization-prepared rapid acquisition gradient echo (MPRAGE) protocol (sagittal slices, 1 mm thickness, 1×1 mm in-plane resolution, TR=11.4 msec, TE=4.4 msec, flip angle=10°).
In all but two participants (whose Run 2 data were excluded due to excessive motion), imaging and behavioral data from two consecutive runs of the verbal learning task were averaged to increase the power of the paradigm.
Image Analysis
Images were analyzed using the AFNI software package. 30 Functional images were aligned across the image time series; time points with remaining visually observable movement after use of the automated algorithm were discarded. Registration between functional and anatomical images was inspected visually and, when necessary, rotated or translated manually into alignment. The time-dependent BOLD signal was modeled using regression with a combination of the following variables: reference vectors representing the occurrence of different stimulus types (i.e., Word pairs or Fixation) convolved with a gamma-function model of the hemodynamic response, motion parameters, a linear trend, and a constant. The magnitudes of the fit coefficient for the general linear contrast in signal intensity (controlling for the other parameters in the model) between Word pairs and Fixation were used as the dependent variables for further analysis. Fit coefficient maps were transformed into standardized atlas space 31 and blurred with an 8 mm FWHM Gaussian filter (for Talairach region of interest [ROI] and whole brain analyses) or left unblurred (for the hippocampal ROI analysis). The fit coefficient maps were then averaged across the two paired-associates learning runs.
Statistical Analyses
Two approaches were utilized to examine our hypotheses regarding the relationship between learning-related brain response and MacCAT-CR performance.
Region of Interest (ROI) Analysis
We calculated the mean learning-related brain response in three bilateral ROIs ( Figure 1 ). Inferior frontal gyrus and middle frontal gyrus ROIs were drawn using the AFNI program DrawDataset, which uses information from the Talairach Daemon software. 32 Following the guidelines of Insausti et al., 33 hippocampal ROIs were hand-traced on each participant’s high-resolution anatomical MR image by two experienced technicians, who achieved >90% agreement on a reliability set of images. The mean fit coefficient in both hemispheres of each ROI was calculated and used in the correlation analysis with MacCAT-CR scores. Pearson’s correlations were used, and considered significant at a two-tailed p≤0.05. Given the exploratory nature of the study and small sample size, no correction for multiple comparisons was made.
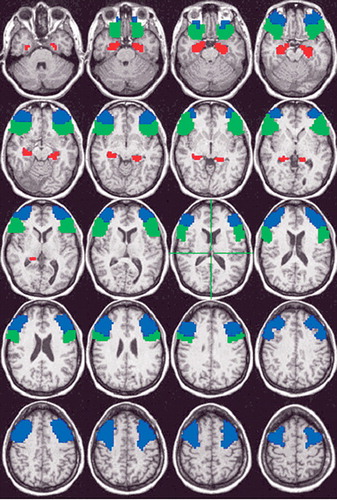
Blue=middle frontal gyrus, Green=inferior frontal gyrus, Red=hand-drawn hippocampal region of interest of a representative participant. ROIs are superimposed on axial slices that span from 25 Inferior to 51 Superior, in 4 mm increments.
Whole Brain Analysis
To see whether other, unhypothesized relationships might be found, the correlation between MacCAT-CR scores and brain response was carried out at each voxel in the entire brain. Clusters of correlated voxels were then identified with the criterion (determined by Monte Carlo simulation) that each significant cluster had to contain at least 160 contiguous voxels, each with a significant correlation (p<0.05). This protected a whole-brain probability of false positive cluster detection of p=0.05.
RESULTS
Participant Characteristics
Patients were middle-aged (mean=44.1 [SD=10.0] years old), high-school educated (12.9 [SD=1.3] years) and predominantly male (93% [N=13]), with a chronic course of illness (duration=22.0 [SD=9.1] years) of moderate severity (PANSS Positive score=10.3 [SD=3.6]; PANSS Negative=14.1 [SD=5.8]; PANSS General=24.2 [SD=5.6]). All were being treated with medications, predominantly with atypical antipsychotics (43% [6] olanzapine, 28% [4] risperidone, 21% [3] other atypicals, 8% [1] typical antipsychotics). Most of the patients were of the paranoid subtype (78% [11]), with the others of the disorganized (14% [2]) or undifferentiated subtypes (8% [1]).
Decisional Capacity
The scores of patients on the Understanding and Reasoning sections of the MacCAT-CR (Initial Understanding [out of 26]: 14.4 [SD=3.8], Trials to Criterion [out of 3]: 1.9 [SD=0.8], Final Understanding [out of 26]: 17.8 [SD=1.7], Reasoning [out of 8]: 6.3 [SD=1.6]) were in the range expected based on previous studies of decisional capacity among patients with schizophrenia. 4 Given that in practice, consent information is generally only presented once, Initial Understanding was used in subsequent analyses.
fMRI Task Performance
Patients were attending well to the stimuli during the scan, as indicated by high accuracy of judgment of capitalization of the words (percent correct: 83.2 [SD=11.5]). Approximately 71% (SD=19.5) of the presented word pairs were recalled correctly after scanning, suggesting that the patients understood the encoding directions and were motivated to try to remember the word pairs.
Correlation Analyses
Regions of Interest
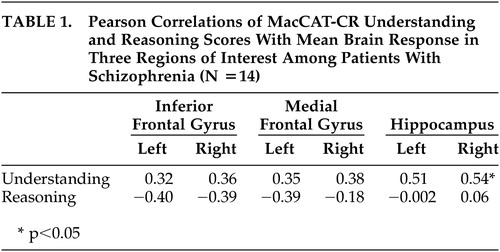
There were no significant correlations between inferior or middle frontal brain response and either Understanding or Reasoning scores ( Table 1 ), but relationships tended to be in the positive direction for Understanding and in the negative direction for Reasoning.
 |
Hippocampal brain response during learning of word pairs was positively correlated to Understanding in the right hemisphere (r=0.54, p=0.04) and there was a trend (r=0.51, p=0.06) toward a relationship in the left hemisphere ( Figure 2 ). No relationship to Reasoning was found in this region.
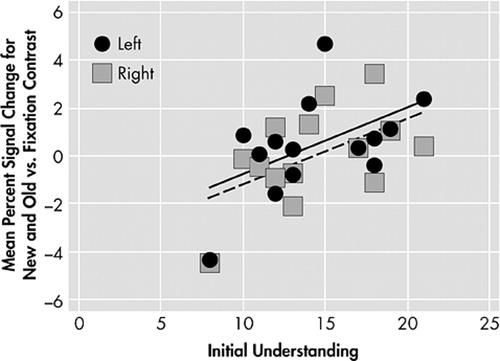
Left hemisphere: solid circles, solid line indicate linear trend. Right hemisphere: open squares, dashed line indicate linear trend.
Whole Brain
Understanding scores were significantly associated with brain response during learning of Word pairs versus Fixation in a large region (349 voxels, all r≥0.53, p≤0.05; center of mass Talairach coordinates: 0.5 right, 34 posterior, 11 inferior), that included bilateral parahippocampal gyrus and midline culmen of the cerebellum, and extended forward into the bilateral thalamus ( Figure 3 ). No clusters of significant relationship between Reasoning scores on the MacCAT-CR and brain response during learning of word pairs were found.
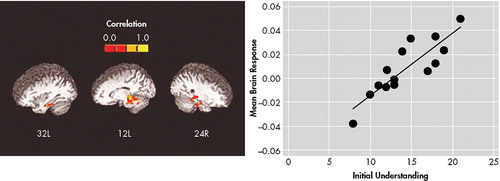
*349 voxels, each with p<0.05. Warmer colors indicate more positive correlations. Inset shows scatter plot of mean brain response in this cluster against mean Initial Understanding score for each patient.
Potential Moderators
We examined the relationship of MacCAT-CR Understanding scores to demographic, clinical, and task performance data to see if any potential confounding variables might be candidates to moderate either of the significant brain response relationships with Understanding. There were no large or significant correlations between Understanding and age, years of education, duration of illness, PANSS scores, or button-press accuracy during scanning. In addition, we examined the possibility that participant motion may have played a moderating role in the observed relationships. Six summary motion scores (three angles of rotation and three directions of translation) were calculated for each participant to index the degree of within-run movement, and the correlation of each with MacCAT-CR Understanding scores was calculated. All of the correlations were small and nonsignificant (range of r=−0.13 to 0.06, df=12), so there was no evidence that those who had lower Understanding moved more.
Consistent with our assumption that understanding of a consent form is related to an individual’s ability to learn and remember information, there was a significant relationship of MacCAT-CR Understanding with the proportion of Word pairs recalled following scanning, such that participants with better Understanding scores also had better word-pair recall (r=0.62, p=0.02, df=12). As mentioned above, right hippocampal activation during the encoding of Word pairs was significantly related to Understanding scores, as was brain response in a large cluster that included the parahippocampus. Activation of these regions was less predictive of postscan Word pair recall (right hippocampus: r=0.40, p=0.15, df=12; parahippocampal cluster: r=0.49, p=0.08, df=12), although the relationships were similarly positive in direction. Presumably, individual differences in the functioning of the brain in these regions underlie the relationship of Understanding scores with word-pair recall and reflect common information processing demands of the two cognitive activities, although a formal moderation analysis would have been underpowered in this preliminary study. Even if moderation was not supported in a larger sample, however, this would not necessarily imply that the association between Understanding and brain response in these regions was unrelated to verbal encoding processes. Unique features of the paired-associates task (e.g., the high imageability of the words) could have brought other brain systems to bear, slightly weakening the association between individual differences in medial temporal brain response and later recall performance compared to the correlation of Understanding (which involves recall of more abstract and complex material) with brain response in these same areas.
DISCUSSION
As predicted, individuals with the best Initial Understanding scores on the MacCAT-CR had the greatest learning-related activation in the hippocampus, a region thought to be crucial for encoding and recognition. 34 Whole brain analyses also revealed that individuals with good initial Understanding scores showed greater learning-related brain response in a region that included bilateral parahippocampal cortex, cerebellum, and thalamus. Activation of these areas has also been observed during verbal learning tasks. 34 These results suggest that good understanding of a consent form relies, at least in part, on adequate engagement of brain systems known to be involved in encoding verbal information.
Contrary to our hypothesis, however, we did not observe a significant relationship of Understanding to inferior prefrontal function. Prefrontal activation during encoding may reflect use of strategies for rehearsal and semantic elaboration of items. 34 Because the consent-related information was presented in a structured way (as part of a narrative), individual differences in Understanding scores might have been less related to strategic encoding processes (as reflected in frontal activation levels) than to more basic consolidation of information into long-term storage (reflected in hippocampal activation levels).
As expected, middle frontal gyrus activation was not related to Understanding, presumably because heterogeneity in Understanding ability is not driven primarily by individual differences in the functioning of areas involved in working memory. However, we also did not find a correlation between Reasoning scores, which we did hypothesize to be related to dorsolateral working memory systems, and brain response of the middle frontal gyrus. It is possible that the lack of association was due to a restriction of range in Reasoning scores. The verbal learning task also may not have been ideally suited to examine the hypothesized relationship with Reasoning, because it involved fewer demands on working memory.
There are several limitations to the present study. First, the sample size of 14 schizophrenia patients was small and restricted to patients who were mostly male and over the age of 40. Thus, we only had power to detect moderate to large associations, and the results may not generalize to younger or female patient groups. In addition, the number of correlations examined raises the risk of type I error. Second, although the MacCAT-CR scores of this sample were similar to other schizophrenia samples, for ethical reasons we were unable to image those patients (N=5) who did not meet criteria for adequate decisional capacity. These individuals might have shown a qualitatively (rather than simply quantitatively) different relationship, which therefore went unobserved. Future studies using a process of surrogate consent could be helpful in this regard. Third, we chose not to examine brain response correlates of appreciation and expression of choice due to the poor psychometric properties of these subscales. Additional studies using scales specifically designed to assess appreciation, such as the California Scale of Appreciation, 8 would allow us to examine hypotheses about neurocognitive systems related to this component. Fourth, our paired-associate learning challenge task, although designed to involve similar cognitive processes and to evoke brain response of hypothetically important regions, was not a close analogue of the informed consent process. A more realistic informed consent task, although challenging to operationalize in the scanner, would likely yield additional information about brain processes related to heterogeneity in decisional capacity. Finally, we did not examine the correlation of brain response and MacCAT-CR scores within a healthy comparison group. Though it would be interesting to investigate whether the observed relationships are also present in healthy individuals, practical limitations in the variability of understanding and reasoning scores would likely make this comparison difficult. Future studies should address the specificity of these findings to schizophrenia relative to other psychiatric disorders that may involve cognitive impairments.
Despite the study limitations, results of the present study suggest that differences in the function of particular brain systems may underlie differences among individuals with schizophrenia in their ability to understand elements of a consent form. What is not known is whether these specific abnormalities can be remediated with focused interventions. For example, pharmacological treatments that target medial temporal brain functioning might be able to improve not only symptoms but also decisional capacity. Furthermore, interventions that aim at improving the informed consent process itself could be developed to focus on strategies to compensate for possible underlying deficits in new learning. Imaging studies using closer analogs of the consent process during scanning could be helpful to specify further the nature of decisional capacity deficits in schizophrenia and suggest possible ways of ameliorating them.
1. National Bioethics Advisory Commission: Research involving persons with mental disorders that may affect decision-making capacity, in Report and Recommendations of the National Bioethics Advisory Commission, vol 1. Rockville, Md, National Bioethics Advisory Commission, 1998Google Scholar
2. Michels R: Are research ethics bad for our mental health? N Engl J Med 1999; 340:1427–1430Google Scholar
3. Wirshing DA, Wirshing WC, Marder SR, et al: Informed consent: assessment of comprehension. Am J Psychiatry 1998; 155:1508–1511Google Scholar
4. Dunn LB, Lindamer LA, Palmer BW, et al: Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am J Geriatr Psychiatry 2002; 10:142–150Google Scholar
5. Carpenter WT Jr, Gold JM, Lahti AC, et al: Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 2000; 57:533–538Google Scholar
6. Palmer BW, Dunn LB, Appelbaum PS, et al: Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Arch Gen Psychiatry 2004; 61:230–236Google Scholar
7. Grisso T, Appelbaum PS: The MacArthur treatment competence study, III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav 1995; 19:149–174Google Scholar
8. Saks ER, Dunn LB, Marshall BJ, et al: The California Scale of Appreciation: a new instrument to measure the appreciation component of capacity to consent to research. Am J Geriatr Psychiatry 2002; 10:166–174Google Scholar
9. Stroup S, Appelbaum P, Swartz M, et al: Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res 2005; 80:1–8Google Scholar
10. Marson DC: Loss of competency in Alzheimer’s disease: conceptual and psychometric approaches. Int J Law Psychiatry 2001; 24:267–283Google Scholar
11. Palmer BW, Jeste DV: Relationship of individual cognitive abilities to specific components of decisional capacity among middle-aged and older patients with schizophrenia. Schizophr Bull 2006; 32:98–106Google Scholar
12. Moser DJ, Schultz SK, Arndt S, et al: Capacity to provide informed consent for participation in schizophrenia and HIV research. Am J Psychiatry 2002; 159:1201–1207Google Scholar
13. Goldberg TE, Weinberger DR: Genes and the parsing of cognitive processes. Trends Cogn Sci 2004; 8:325–335Google Scholar
14. Saykin AJ, Gur RC, Gur RE, et al: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Google Scholar
15. Paulsen JS, Heaton RK, Sadek JR, et al: The nature of learning and memory impairments in schizophrenia. J Int Neuropsychol Soc 1995; 1:88–99Google Scholar
16. Eyler Zorrilla L, Jeste DV, Brown GG: Functional MRI and novel picture learning among older patients with chronic schizophrenia: abnormal correlations between recognition memory and medial temporal brain response. Am J Geriatr Psychiatry 2002; 10:52–61Google Scholar
17. Eyler Zorrilla L, Jeste DV, Paulus MP, et al: Functional abnormalities of medial temporal and frontal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res 2002; 59:187–198Google Scholar
18. Ragland JD, Gur RC, Raz J, et al: Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry 2001; 158:1114–1125Google Scholar
19. Ragland JD, Gur RC, Glahn DC, et al: Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology 1998; 12:399–413Google Scholar
20. Gur RE, Jaggi JL, Shtasel DL, et al: Cerebral blood flow in schizophrenia: effects of memory processing on regional activation. Biol Psychiatry 1994; 35:3–15Google Scholar
21. Leube DT, Rapp A, Erb M, et al: Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia: an fMRI study. Schizophr Res 2003; 64:83–85Google Scholar
22. Gold JM, Carpenter C, Randolph C, et al: Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 1997; 54:159–165Google Scholar
23. Callicott JH, Bertolino A, Mattay VS, et al: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Google Scholar
24. Manoach DS, Gollub RL, Benson ES, et al: Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Google Scholar
25. Barch D, Csernansky JG, Snydert A, et al: Dorsolateral prefrontal cortex dsyfunction in schizophrenia: relationship to both working memory and long term memory. Neuroimage 2000; 11:S193Google Scholar
26. Eyler Zorrilla LT, Heaton RK, McAdams LA, et al: Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: no differences in age-related cognitive decline. Am J Psychiatry 2000; 157:1324–1326Google Scholar
27. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Press, 1994Google Scholar
28. Appelbaum P, Grisso T: MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Sarasota, Fla, Professional Resource Press, 2001Google Scholar
29. Stroup TS, McEvoy JP, Swartz MS, et al: The National Institute of Mental Health clinical antipsychotic trials of intervention effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 2003; 29:15–31Google Scholar
30. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Google Scholar
31. Talairach J, Tournoux P: A Co-Planar Stereotaxic Atlas of the Human Brain. New York, Theime, 1988Google Scholar
32. Lancaster JL, Woldorff MG, Parsons LM, et al: Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10:120–131Google Scholar
33. Insausti R, Juottonen K, Soininen H, et al: MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol 1998; 19:659–671Google Scholar
34. Cabeza R, Nyberg L: Imaging cognition II: an empirical review of 275. PET and fMRI studies. J Cogn Neurosci 2000; 12:1–47Google Scholar



