Prediction of Clinical Outcomes From rTMS in Depressed Patients With Lateral Visual Field Stimulation: A Replication
Schiffer et al. 9 reported that affective responses to baseline lateral visual field stimulation predicted the clinical responses to a 2-week course of rTMS in 37 patients with a history of chronic major depression. In essence, the authors predicted that patients with a positive left hemispheric emotional valence by lateral visual field stimulation would do well with a treatment that stimulated the left hemisphere and that patients with a negative left hemispheric emotional valence by lateral visual field stimulation would do poorly with this treatment. In that study, the results showed that among 20 patients predicted to do well, the mean reduction in their Hamilton Depression Rating Scale (HAM-D) scores from baseline to 2 weeks posttreatment was 42%, while those 15 patients predicted to do poorly had only an 11% improvement. Seventy-five percent of those predicted to do well had greater than a 20% improvement in their HAM-D scores at 2 weeks posttreatment, and 80% of those predicted to do poorly had less than a 20% improvement.
In the present study, we attempted to replicate Schiffer’s rTMS findings. 9 Based on work by Wittling and colleagues 10 , 11 that reported subjects’ differential physiological and emotional responses to videos shown to one visual field versus the other, Schiffer 12 found in a placebo-controlled study that 60% of 70 patients in a psychotherapy practice reported at least a 20% difference on a simple anxiety measure between sides. As Wittling and Schweiger 11 reported, Schiffer found that the side that evoked more anxiety varied among subjects. EEG, ear temperature, 13 and fMRI studies 14 indicated that lateral visual field stimulation activated the contralateral hemisphere. Thus, the combination of lateralized affective and physiological responses suggest that this technique may reveal something about the laterality of emotional processing in an individual subject that may influence his or her response to a lateralized treatment for depression.
In our 2002 report, 9 we showed a scatter plot that identified male and female subjects, but we did not report gender differences because we felt our sample was too small. In a reevaluation of that data, the 2-tailed Pearson’s correlation for men (N=11) between the measures of depression in response to lateral visual field stimulation and HAM-D percent change at 2 weeks posttreatment was strong (r=0.84, p=0.0011), whereas for women (N=26) it was weaker (r=0.26, p=0.19, nsec). In the present study, we sought to replicate the general finding that lateral visual field stimulation predicted responses to left-sided rTMS as well as to ascertain if these gender differences in predictive response held in an independent sample.
METHOD
The procedures were performed at MindCare Centres, Vancouver, British Columbia, Canada, a clinic that specializes in the treatment of depression with rTMS. In a chart review study, we evaluated data from 23 consecutive patients who were treated at the clinic for depression with left-sided rTMS. Prior to the evaluation or treatment, each patient was offered an explanation of the procedures and asked to give signed informed consent. Twelve subjects (six women) were diagnosed with major depression based on the Structured Clinical Interview for DSM-III-R. 15 Four subjects (three women) were diagnosed with major depression and a generalized anxiety disorder, and four subjects (three women) were diagnosed with dysthymic disorder. Two women were diagnosed with bipolar affective disorder II and one man with bipolar affective disorder I. Two patients (one woman) were left-handed and the mean age of the 14 women was 51 years (SD=9). For the nine men the mean age was 51 (SD=18). All patients were screened for contraindications to rTMS therapy, such as a history of a seizure disorder or ferromagnetic material in the cranium. Each patient filled out a Beck Depression Inventory (BDI) 16 prior to rTMS and at discharge from the clinic 2 weeks later.
Lateral Visual Field Stimulation
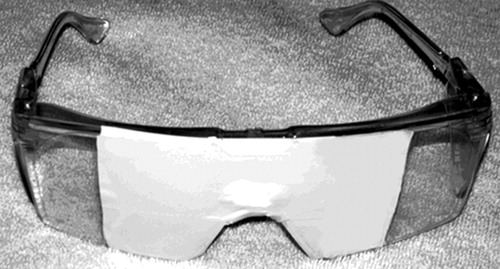
Added to the standard evaluation was lateral visual field stimulation, which consisted of asking patients to wear taped goggles, as shown in Figure 1 , while looking out of either the left or right visual field, chosen randomly. After 45 seconds, while still looking out of the given visual field, the patient was asked to rate his immediate, present state of depression from 0 (none) to 10 (extreme). Then the patient was asked to look out of the opposite visual field and again after 45 seconds was asked to rate his state of depression on the same scale. Patients were asked to look so that the glasses occluded all of one eye and the medial half of the other by fixating on the edge of the tape on the side from which they were looking.
Rapid Transcranial Magnetic Stimulation (rTMS)
Treatment was administered by a trained rTMS technician blind to the lateral visual field stimulation testing and overseen by a medical doctor. A Medtronic Magpro X100 stimulator unit delivered the rTMS, employing a figure-8 butterfly coil held against the scalp, targeting the dorsolateral prefrontal cortex at 10 Hz with 50 stimuli per train, a 20 second intertrain interval, and an intensity of 120% of motor threshold for 50 trains. Two sessions of rTMS were administered each day over a period of 10 days.
Statistical Methods
Because we were attempting to replicate a previous study, we used one-sided tests of significance to evaluate our Pearson’s correlations between the rTMS results and the baseline lateral visual field stimulation. Kolmogorov-Smirnov tests showed that both variables were normally distributed (p>0.5 in both cases). Analysis of covariance (ANCOVA) was used to evaluate the influence of age, handedness, diagnostic category, and initial BDI on the relation between the rTMS results and the baseline lateral visual field stimulation measures. Data are presented as mean±standard deviation.
RESULTS
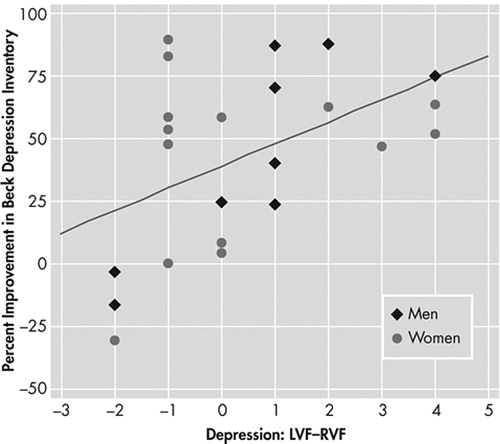
For all 23 patients there was a significant Pearson’s correlation between the percent improvement in BDI from initial score to discharge score and the intensity of depression rated by patients as they looked out the left visual field (LVF) minus that from their right visual field (RVF) (r=0.48, p<0.01). Neither the initial BDI (p>0.6), age (p>0.6), diagnostic category (p>0.13), nor handedness (p>0.33) acted as a significant covariate with lateral visual field stimulation in predicting the outcomes to the rTMS. For the nine men, this correlation was strong (r=0.82, p<0.005), and for the 14 women it was weaker (r=0.27, p=0.18, nsec). Figure 2 shows these data graphically. Table 1 shows the comparable correlations from the 2002 study 9 alongside those from the present study, and shows almost identical correlation values for the data from the two independent studies.
*Measured as the percent improvement between the initial BDI and the BDI after the end of treatment
LVF−RVF >0 suggests a right negative hemispheric emotional valence
 |
In the present study, the group predicted to do well (LVF−RVF>0, N=10) had a 60.8%±20.6 decrease in BDI from initial to discharge score while those with LVF−RVF<0 (N=9) had a decrease in BDI of 31.2%±44.4. This difference was significant by a one-tailed t test (t=−1.83, df=11, p=0.047). The hypothesis that subjects who had higher depression ratings when looking out left visual field than right visual field (LVF−RVF>0) would have a positive response to left-sided transcranial magnetic stimulation (TMS) 10 , 11 was fulfilled in all 10 cases ( Figure 2 ). However, the hypothesis that subjects with LVF−RVF<0 would do poorly with left-sided TMS failed in five of nine cases. Interestingly, all of the predictive failures occurred in women and all were less than 55 years old (42.6±6.5 years). There was a strong correlation between lateral visual field stimulation and change in BDI (r=0.83, N=13, p<0.0003) for men and women 55 years or older. There was a weak correlation between lateral visual field stimulation and percent change in BDI for women less than 55 years old (r=0.07, p>0.8, nsec). Two subjects (one man) were left-handed and their responses to rTMS were predicted by lateral visual field stimulation.
Nine subjects had at least a 2-point difference between left visual field and right visual field on lateral visual field stimulation. Of these, six (two men) had a positive difference and three (two men) had a negative difference, and all had responses to rTMS that were correctly predicted by the lateral visual field stimulation. For these nine subjects, the correlation between BDI percent change and lateral visual field stimulation (LVF–RVF) depression scores was quite high (r=0.88, p=0.0008).
DISCUSSION
In this study we were able to replicate the overall and within gender correlations that we reported in 2002. 9 In both studies, patients with a relative right negative hemispheric emotional valence (reporting more intense feelings of depression while looking out of the left visual field than the right visual field) tended to do well in response to left-sided rTMS at 10 Hz. In the present study, those who did not follow the predictions were women predicted to do poorly by lateral visual field stimulation, and of these five, all were younger than 55 years. Transcranial magnetic stimulation studies 17 , 18 indicate that menstrual cycle hormonal changes can affect the corpus callosal transit time which would be expected to alter the predictability of lateral visual field stimulation. Throughout the literature on laterality, there has been an impression that for many tasks women may be less lateralized than males, 19 – 21 and if true, this premise may relate to our findings. However, lateral dichotic listening tests predicted the response to fluoxetine in females, 22 so the meaning of our gender findings will need further exploration.
Regardless of the theoretical basis for our findings, the idea—that we have been able to predict in two studies which males would respond to left-sided rTMS—represents an important step in improving the efficacy of rTMS. A next step would be to see if subjects who are predicted by lateral visual field stimulation to do well in response to right-sided rTMS do so. If so, the efficacy of rTMS would be markedly improved.
Speer et al. 23 studied 10 adult patients with major depression who were treated with two trials of TMS, one at 20 Hz and one at 1 Hz. In addition to outcome measures, they studied each patient with baseline positron emission tomography (PET) scans that were repeated after each of the two 10-day treatment protocols. They found that subjects who responded to 20 Hz did poorly with 1 Hz and that subjects who responded to 1 Hz did poorly with 20 Hz. The PET scans showed the largest changes in the left hemisphere in the prefrontal cortex, cingulate, and amygdala. On average these areas showed increased activity following the 20 Hz treatment and decreased activity following the 1 Hz treatment. This study suggests that patients seem to be divided into two groups, one that benefited from left sided activation and one that did not. A future study might investigate whether those who responded to the left-sided 20 Hz course of treatment would feel improvement when they look out of the right visual field compared to the left visual field after lateral visual field stimulation, and whether those who responded to the 1 Hz treatment would respond to lateral visual field stimulation with more improvement in affect when looking out of the left visual field compared to the right visual field.
Kimbrell and associates 24 also found that patients who responded to left-sided TMS at 20 Hz tended to do poorly with 1 Hz stimulation and vice versa. Those who responded better to the 20 Hz stimulation tended to have a relative hypoperfusion of generalized cerebral blood flow by baseline PET. Inspection of the authors’ data suggests that the baseline hypoperfusion was greatest in left anterior sites, especially the left anterior cingulate. The authors presented a case of the one patient who received PET scans after treatment as well as before. This patient, a 46-year-old man with refractory unipolar depression with marked baseline left-hemispheric hypoperfusion, had his perfusion normalized after clinically successful TMS at 20 Hz. This patient did poorly with 1 Hz. We feel that future studies would do well to include baseline lateral visual field stimulation measurements to see if baseline imaging measures and outcomes to TMS bear a relation to it.
Mottaghy and colleagues 25 used single photon emission tomography (SPECT) to predict subsequent clinical responses to left-sided rTMS in 17 patients. When a corrected threshold was used, baseline SPECT activity in the right periinsular cortex correlated positively with the rTMS outcomes.
Eschweiler et al. 26 used multisite near-infrared spectroscopy during an arithmetic and a left and a right mirror drawing task to predict the response to rTMS. The left-handed mirror drawing task and a total hemoglobin, but neither oxy nor deoxy-hemoglobin at F3 but not other sites, were able to predict which four of the 12 patients would have a greater than 30% reduction in HAM-D following left-sided rTMS. Compared with SPECT, PET, and near-infrared spectroscopy, lateral visual field stimulation is inexpensive and convenient.
A number of authors have asserted that hypoactivity of the left frontal cortex during baseline states is associated with depression, but this hypothesis has been inconsistently supported by the literature. For instance, Henriques and Davidson 27 have suggested that depression is associated with relatively less left frontal activity by alpha EEG, but this finding has often not been replicated. 28 , 29 Some authors 30 , 31 but not others 32 , 33 have drawn a similar conclusion using functional imaging. Robinson et al. 34 have argued that left frontal strokes are more often associated with depression than strokes from other areas, but this assertion is countered by Carson and his associates. 35 Some studies, 36 , 37 but not others, 38 – 40 have shown increased left frontal activation after successful treatment for depression with antidepressants or psychological treatment. Studies correlating left rTMS treatments with increases in left frontal cerebral blood flow have reported a significant relationship, 25 , 41 but a relationship has not been found between positive outcomes from rTMS and increases in left frontal activation when comparing before and after a treatment course. 25 , 41 We have argued 1 that some of the inconsistencies in research involving cerebral laterality may be due to the tendency for researchers to average their data without regard to hemispheric emotional valence, and we suggested 1 that by including hemispheric emotional valence as a baseline variable in the evaluation of such data, such findings might be further clarified. Although lateral visual field stimulation has been found by blood-oxygen-level-dependent (BOLD) fMRI 14 and EEG 13 to activate the contralateral hemisphere, we have not yet studied whether the side of negative hemispheric emotional valence correlates with alterations in cerebral frontal activity.
We reported 12 that among psychotherapy patients who had lateralized affective responses to lateral visual field stimulation, 73% of 15 patients with major depression had a right negative hemispheric emotional valence, and among 14 with posttraumatic stress disorder (PTSD), 71% had a left negative hemispheric emotional valence. Cohen et al. 42 reported the results of a double-blind, controlled trial of right-sided rTMS among 24 patients with PTSD. They found that these patients had highly significant reductions in PTSD symptoms in response to TMS over the right dorsolateral prefrontal cortex at a stimulating frequency of 10 Hz compared with a 1 Hz inhibitory frequency or with a sham condition. The fact that the positive right hemispheric emotional valences that we found in our patients with PTSD and Cohen’s results are consistent our hypothesis about rTMS and laterality could be coincidence but does deserve serious further study because of the potential benefit to patients with this disorder. To date no adequate hypothesis has been put forth to explain the benefits of rTMS, 43 and so the investigation of even novel hypotheses should be undertaken.
Our findings may apply as well to other lateralized treatments such as ECT. There is evidence that psychotropic medications have lateralized effects, 44 , 45 and several authors have predicted responses to psychotropic medications by measurement of asymmetric brain activation by dichotic listening, 22 , 46 EEG, 46 , 47 fMRI, 48 and PET. 49 We feel that lateral visual field stimulation should be explored as a possible method for predicting such outcomes.
1 . Schiffer F, Teicher M, Anderson C, et al: Determination of hemispheric emotional valence in individual subjects: a new approach with research and therapeutic implications. Behav Brain Funct 2007; 3:13Google Scholar
2 . Davidson R: Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology 2003; 40:655–665Google Scholar
3 . Joseph R: Neuropsychology, Neuropsychiatry, and Behavioral Neurology. New York, Plenum Press, 1990Google Scholar
4 . Robinson RG, Starkstein SE, Price TR: Post-stroke depression and lesion location. Stroke 1988; 19:125–126Google Scholar
5 . Schore A: Early organization of the nonlinear right brain and development of a predisposition to psychiatric disorders. Dev Psychopathol 1997; 9:595–631Google Scholar
6 . Seigal D: The Developing Mind. New York, Guilford, 1999Google Scholar
7 . Papanicolaou A, Johnstone J: Probe evoked potentials: theory, method, and applications. Int J Neurosci 1984; 24:107–131Google Scholar
8 . Schiffer F, Teicher M, Papanicolaou A: Evoked potential evidence for right brain activity during the recall of traumatic memories. J Neuropsychiatry Clin Neurosci 1995; 7:169–175Google Scholar
9 . Schiffer F, Stinchfield Z, Pascual-Leone A: Prediction of clinical response to transcranial magnetic stimulation for depression by baseline lateral visual stimulation. Neuropsychiatry Neuropsychol Behav Neurol 2002; 15:18–27Google Scholar
10 . Wittling W, Roschmann R: Emotion-related hemisphere asymmetry: subjective emotional responses to laterally presented films. Cortex 1993; 29:431–448Google Scholar
11 . Wittling W, Schweiger E: Neuroendocrine brain asymmetry and physical complaints. Neuropsychologia 1993; 31:591–608Google Scholar
12 . Schiffer F: Affect changes observed with right versus left lateral visual field stimulation in psychotherapy patients: possible physiological, psychological, and therapeutic implications. Compr Psychiatry 1997; 38:289–295Google Scholar
13 . Schiffer F, Anderson C, Teicher M: EEG, bilateral ear temperature, and affect changes induced by lateral visual field stimulation. Compr Psychiatry 1999; 40:221–225Google Scholar
14 . Schiffer F, Mottaghy F, Vimal RP, et al: Lateral visual field stimulation reveals extrastriate cortical activation in the contralateral hemisphere: an fMRI study. Psychiatry Res 2004; 131:1–9Google Scholar
15 . Spitzer RL, Williams JB, Gibbon M, et al: The structured clinical interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Google Scholar
16 . Beck A, Ward C, Mendelson M, et al: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Google Scholar
17 . Hausmann M, Tegenthoff M, Sanger J, et al: Transcallosal inhibition across the menstrual cycle: a TMS study. Clin Neurophysiol 2005; 117:26–32Google Scholar
18 . Cahn S, Herzog A, Pascual-Leone A: Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. J Clin Neurophysiol 2003; 20:371–374Google Scholar
19 . Kansaku K, Yamaura A, Kitazawa S: Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex 2000; 10:866–872Google Scholar
20 . Kimura D: Sex and Cognition. London, MIT Press, 1999Google Scholar
21 . Shaywitz B, Shaywitz S, Pugh K, et al: Sex differences in the functional organization of the brain for language. Nature 1995; 373:607–609Google Scholar
22 . Bruder G, Stewart J, McGrath P, et al: Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neuropsychopharmacology 2004; 29:1752–1761Google Scholar
23 . Speer A, Kimbrell T, Wassermann E, et al: Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000; 8:1133–1141Google Scholar
24 . Kimbrell TA, Little JT, Dunn RT, et al: Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999; 46:1603–1613Google Scholar
25 . Mottaghy F, Keller C, Gangitano M, et al: Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res Neuroimaging 2002; 115:1–14Google Scholar
26 . Eschweiler G, Wegerer C, Schlotter W, et al: Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res 2000; 99:161–172Google Scholar
27 . Henriques J, Davidson R: Left frontal hypoactivation in depression. J Abnorm Psychol 1991; 100:535–545Google Scholar
28 . Vuga M, Fox N, Cohn J, et al: Long-term stability of frontal electroencephalographic asymmetry in adults with a history of depression and controls. Int J Psychophysiol 2006; 59:107–115Google Scholar
29 . Hagemann D: Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biol Psychol 2004; 67:157–182Google Scholar
30 . Bench C, Friston K, Brown R, et al: The anatomy of melancholia–focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607–615Google Scholar
31 . Baxter LJ, Schwartz J, Phelps M, et al: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Google Scholar
32 . Drevets W, Videen T, Price J, et al: A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628–3641Google Scholar
33 . Hurwitz T, Clark C, Murphy E, et al: Regional cerebral glucose metabolism in major depressive disorder. Can J Psychiatry 1990; 35:684–688Google Scholar
34 . Morris PL, Robinson RG, Raphael B, et al: Lesion location and poststroke depression. J Neuropsychiatry Clin Neurosci 1996; 8:399–403Google Scholar
35 . Carson AJ, MacHale S, Allen K, et al: Depression after stroke and lesion location: a systematic review. Lancet 2000; 356:122–126Google Scholar
36 . Kennedy S, Evans K, Kruger S, et al: Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158:899–905Google Scholar
37 . Navarro V, Gasto C, Lomena F, et al: Normalization of frontal cerebral perfusion in remitted elderly major depression: a 12-month follow-up SPECT study. Neuroimage 2002; 16:781–787Google Scholar
38 . Brody A, Saxena S, Silverman D, et al: Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res 1999; 91:127–139Google Scholar
39 . Buchsbaum M, Wu J, Siegel B, et al: Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 1997; 41:15–22Google Scholar
40 . Goldapple K, Segal Z, Garson C, et al: Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004; 61:34–41Google Scholar
41 . Catafau A, Perez V, Gironell A, et al: SPECT mapping of cerebral activity changes induced by repetitive transcranial magnetic stimulation in depressed patients: a pilot study. Psychiatry Res 2001; 106:151–160Google Scholar
42 . Cohen H, Kaplan Z, Kotler M, et al: Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2004; 161:515–524Google Scholar
43 . Padberg F, Moller H: Repetitive transcranial magnetic stimulation: does it have potential in the treatment of depression? CNS Drugs 2003; 17:383–403Google Scholar
44 . Ingum J, Bjorklund R: Effects of flunitrazepam on responses to lateralized visual stimuli: evidence for cerebral asymmetry of execution of manual movements to targets in contralateral and ipsilateral visual space. Psychopharmacology (Berl) 1994; 114:551–558Google Scholar
45 . Tomer R: Neuroleptic effects on interhemispheric and intrahemispheric performance of tactile discrimination tasks by schizophrenic patients. Psychiatry Res 1990; 32:289–296Google Scholar
46 . Bruder G, Stewart J, Tenke C, et al: Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biol Psychiatry 2001; 49:416–425Google Scholar
47 . Knott V, Labelle A, Jones B, Mahoney C: EEG hemispheric asymmetry as a predictor and correlate of short-term response to clozapine treatment in schizophrenia. Clin Electroencephalogr 2000; 31:145–152Google Scholar
48 . Davidson R, Irwin W, Anderle M, et al: The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160:64–75Google Scholar
49 . Little JT, Ketter TA, Kimbrell TA, et al: Bupropion and venlafaxine responders differ in pretreatment regional cerebral metabolism in unipolar depression. Biol Psychiatry 2005; 57:220–228Google Scholar