Cognitive Dysfunctions Associated With PTSD: Evidence from World War II Prisoners of War
There are several plausible reasons for inconsistency in the identified cognitive dysfunctions. First, the studied PTSD populations can have confounding comorbidities, each with its own associated cognitive dysfunctions. The most common comorbidities include, but are not limited to, chronic alcohol and substance abuse, previous head injuries, and the presence of other psychiatric conditions. Alcohol abuse has been associated with impaired executive function, 5 , 6 visuospatial processing, working memory, 7 and/or anterograde and retrograde memory, all of which could falsely be attributed to PTSD if a study population has significant alcohol use. In addition, Vietnam-era veterans have been reported to “self-medicate” with alcohol, marijuana, heroin, and benzodiazepines to ease the PTSD symptoms. 8 , 9 Each of these substances has associated cognitive deficits; however, inconsistent and intermittent use of each substance in this population is typical, resulting in either those who have variable cognitive impairments or those who fluctuate in their degree of drug use. 10 Closed or open head injury with loss of consciousness has been associated with deceleration-acceleration injuries, diffuse axonal injury, hemorrhage/contusion, infarction, and focal traumatic lesions in the case of open head injury; each could lead to a different cognitive dysfunction. 11 – 13 Head injury is common among combat veterans with PTSD, and those with a history of head injury report higher levels of PTSD symptoms than those without a history of head injury. 14
The presence of comorbid psychiatric diagnoses appears to have a large impact on cognitive function as well. Brady et al. 15 showed that a substantial number of PTSD patients have comorbid psychiatric diagnoses including substance abuse, depression, and anxiety disorders. Major depressive disorder has been associated with cognitive impairments, 16 and since a current or lifetime history of major depression may be present in a majority of patients with combat-related PTSD, 17 identifying the cognitive dysfunction associated with PTSD would have to account for these depression-related impairments.
Another factor that has obscured identifying the cognitive dysfunctions specifically associated with PTSD is determining whether deficits existed prior to, or developed secondary to, PTSD. Two key examples of this are IQ and memory. Vasterling et al. 18 suggested that after a comparable traumatic experience, one of the differences between those who acquire PTSD and those who do not is IQ—those with lower relative IQs appear to be at increased risk of developing PTSD. Brandes et al. 2 tested individuals within 10 days after a traumatic event, and those meeting criteria for PTSD had lower IQs, suggesting that IQ differences between subjects with and without PTSD either were premorbid in nature or occur very early in the course of PTSD. There are other factors that need to be considered before concluding that lower IQ is a predisposing condition for the development of PTSD. Sutker et al. 19 studied former Korean and World War II prisoners of war (POWs) and evaluated cognitive performance by groups (defined by weight losses of greater than 35% of premorbid weight, or less than or equal to 35%) and non-POW combat veterans. Percent weight loss is considered a physical marker of stress in prisoners, particularly if the prisoners were not starved. Sutker et al. 19 found that POWs with significant weight loss performed more poorly than combat veterans on subtests of the WAIS and Wechsler Memory Scale (WMS), suggesting that the severity of stress reflected by trauma-induced weight loss played a role in WAIS performance, making low WAIS IQ less likely a premorbid condition.
It is unclear which of the cognitive impairments reported in PTSD are genuinely related to developing PTSD, pretrauma risk factors, or both. The presence of comorbidities with their own associated cognitive deficits in those with PTSD, not all of which are delineated in each study, left interpretation of the previous findings difficult. In order to minimize these difficulties in data interpretation, we studied a group of former World War II POWs with comparable traumatic experiences, yet minimal comorbidities, and compared cognition in those who developed PTSD with those who did not.
METHOD
Subjects
A total of 25 male former POW subjects participated in the study. The Human Use Committee of the University of Arkansas for Medical Sciences approved the research protocol, and written informed consent was obtained from all subjects. All POW subjects both had combat experience and had been held captive during either World War II or the Korean War. Eight of the 10 former POWs with PTSD were German POWs and two were Japanese POWs. Seven of the 10 former POWs without PTSD were German POWs, two were Japanese POWs, and one was a Korean POW. All study subjects were carefully selected using stringent exclusion and inclusion criteria. All subjects were right-handed, had no history of traumatic brain injury with loss of consciousness, had no history of neurological impairment or degenerative neurological illness, did not meet criteria for either current or lifetime alcohol dependence or substance abuse, and had Mini-Mental State Exam (MMSE) scores of 26 or higher. All research subjects volunteered to complete the study without any compensation.
The subjects were recruited through a POW outreach program initiated by the Central Arkansas Veteran’s Health Care System. Only four subjects had a history of any previous psychiatric treatment; all four were POWs with PTSD. No study subject had a history of psychiatric hospitalization, alcohol or substance abuse treatment, or history of suicide attempt. The presence or absence of PTSD was ascertained with the use of the Clinician-Administered PTSD Scale (CAPS-2) for all subjects. 20 CAPS-2 scores were tallied for all subjects in terms of both total score and symptom cluster scores (re-experiencing, avoidance, and arousal). With the CAPS-2, the more conservative “rule of 4” was used 21 —i.e., the frequency and severity scores needed to meet symptom criteria had to add to a minimum of four. The Structured Clinical Interview for DSM-IV 22 was also administered to determine current and lifetime psychiatric diagnoses. Subjects were initially interviewed by a board-certified psychiatrist (TWF or TK) and their medical histories were also reviewed (LB). Military medical treatment files were reviewed to ascertain individual lengths of confinement and pre- and postconfinement body weights (LB). Based on diagnostic interview findings, POW subjects were divided into three groups: POWs with no PTSD or psychiatric diagnoses; POWs with PTSD and psychiatric comorbidities (depression, panic disorder, phobia, generalized anxiety disorder); and POWs with PTSD only.
Neuropsychological Tests
The neuropsychological battery of tests for this population included the following:
| • | Mini-Mental State Exam 23 | ||||
| • | North American Adult Reading Test (NAART)—used as an estimate of IQ 24 | ||||
| • | Boston Naming Test 25 | ||||
| • | Category Fluency 26 | ||||
| • | |||||
| • | |||||
| • | Digit span forward and backward (WAIS-III) 31 | ||||
| • | Rey Auditory Verbal Learning Test 32 | ||||
| • | Logical memory subtest of the WMS-R 33 | ||||
| • | Warrington Recognition Memory Test (Faces) 34 | ||||
| • | Judgment of line orientation 35 | ||||
| • | |||||
| • | Symbol Digit Modalities Test 36 | ||||
| • | |||||
Procedures
The patients were tested in one of two dedicated research neuropsychological testing rooms with door insulation for noise reduction, comfortable seating, a testing table, no intercom or external speakers, and signs to designate that testing was in session. Testing was performed by trained neuropsychological testing technicians. The testing was performed in single sessions totaling approximately 80–90 minutes. The technician recorded all subject responses and scored them after the testing sessions. These responses were entered into a spreadsheet for further data analysis.
RESULTS
The data were entered into SPSS statistical analysis package (SPSS Inc., Chicago). The initial cross-sectional analyses across groups revealed several key findings ( Table 1 ), including the absence of significant group differences in age or education. A one-factor independent measures analysis of variance (ANOVA) on the North American Adult Reading Test IQ measure, grouped by POW diagnosis (no PTSD, PTSD only, PTSD + psychiatric comorbidities), was significant (F=4.59, df=2, 18, p=0.02). Post hoc t tests comparing groups to the population at large revealed a significant difference of IQ score of POWs without PTSD (t=2.99, df=7, p<0.05) and no significant findings with either PTSD group.
 |
One-way ANOVA with all three groups for the neuropsychological test data revealed a significant group difference on Symbol Digit Modalities Test performance. Group performance was significantly less for those with PTSD only and demonstrated a trend toward a difference for the group with PTSD+psychiatric comorbidities ( Table 2 ).
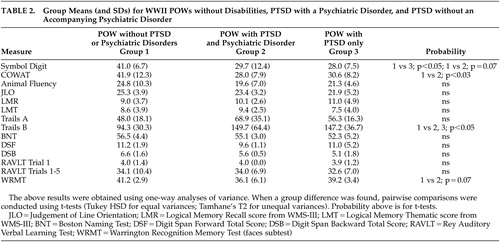 |
Phonemic fluency (as measured by results on the Controlled Oral Word Association Test) was significantly less for the group with PTSD+psychiatric comorbidities for the total fluency score. It is of interest that animal fluency, more a measure of semantic processing than fluency, was not impaired for these groups ( Table 2 ).
Trails B performance was significantly slower for both the PTSD+psychiatric comorbidities and PTSD only groups compared to those without PTSD ( Table 2 ). Trails A performance was not different between any of the three groups.
There was a trend toward significance of recognition memory for faces in the PTSD + psychiatric comorbidities group ( Table 2 ).
Correlations were assessed for variables associated with trauma, such as percent weight loss to pre-POW weight, number of months in combat, number of months as a POW, and total score on the Clinician-Administered PTSD Scale, as well as neuropsychological performance (see Table 3 ). Percent weight loss correlated significantly with age, months of combat, months as a POW, total score on the Clinician-Administered PTSD Scale, as well as the neuropsychological variables of Symbol Digit, phonemic fluency (Controlled Oral Word Association Test), and recognition memory for faces. It is important to note that North American Adult Reading Test IQ did not correlate with the markers of trauma (percent weight loss, months of combat, months as a POW, and total score on the Clinician-Administered PTSD Scale). A logistic regression analysis revealed that 29% of the probability of a diagnosis of PTSD could be accounted for by an individual’s IQ estimate (p<0.02). If percent weight loss during POW confinement is included as a variable in the logistic regression, then IQ estimate and percent weight loss together account for 40% of the variance of the probability of a PTSD diagnosis ( N=21; χ 2 =11.159, p<0.004, df=17).
 |
DISCUSSION
As noted, it has been difficult to determine if there are specific cognitive dysfunctions associated with PTSD, and if so, whether these were precursors to, and/or are associated with, developing PTSD. This group of POWs provides a unique study of individuals with PTSD having minimal confounds and comorbidities. Additionally, this group of subjects was clearly exposed to severe trauma during early adulthood and had documented objective measurements of this trauma (e.g., weight loss, time of imprisonment) recorded at that time.
A major finding of the study was that IQ was significantly higher in those who did not develop PTSD compared to those who did. This was the case even when controlling for factors such as weight loss, time of imprisonment, and time as a POW. Given that the comparison group in this study was veterans who experienced comparable trauma, these differences in IQ cannot be attributed to the trauma alone. Since subjects with a history of head injury were excluded, head injury could not possibly account for such a difference. The most compelling finding was that premorbid higher IQ was protective against developing PTSD. While the other cognitive deficits that were detected had a significant association with markers of the traumatic event, IQ performance did not correlate with any of these factors: percent weight loss, months of combat, or months as a POW. Thus, the primary finding that higher IQ may protect people who experience a trauma from developing PTSD appears to be unrelated to the degree of trauma.
Symbol Digit, a marker of visual scanning and psychomotor speed, 39 and Trails B appear to be associated with PTSD in general, regardless of the presence or absence of psychiatric comorbidities (Symbol Digit significant for POWs with PTSD only and a trend for POWs with PTSD+ psychiatric comorbidities; Trails B significant for both). Psychomotor speed is a generalized, nonspecific cognitive impairment that does not necessarily imply pathologic changes in a focal anatomic structure, and it is unclear from the proposed models of pathogenesis in PTSD how this psychomotor dysfunction would develop. However, unlike IQ, this deficit is significantly correlated with factors relating to the traumatic event (e.g., percent weight loss) and is thus unlikely to be a premorbid or predisposing condition to developing PTSD. A relative decrement in performance on Trails B without a corresponding decrement for Trails A was noted in both PTSD groups. Isolated Trails B without Trails A decrements suggest that the psychomotor component present in both tasks is not likely to be the etiology of the decreased performance, but the source is the additional frontal lobe component of processing alternating sequences required in Trails B. 40 , 41 While these two frontal lobe functions appear affected in PTSD, several other frontal lobe tests administered (digit span backward, category fluency) remain unaffected, further demonstrating the selectivity in frontal lobe disruption in PTSD. 4 The anatomic localization associated with Trails B performance has not been clearly identified in general and thus cannot be correlated to specific frontal regions in PTSD.
There are deficits associated only with those POWs with PTSD + psychiatric comorbidities and not with just PTSD alone. These are focal cognitive dysfunction in phonemic fluency and a trend toward a decrement in recognition memory for faces. While phonemic fluency is decreased for this group, it is of interest that animal fluency, more indicative of semantic processing compared to phonemic fluency, is not impaired in this group. In functional imaging studies, phonemic fluency has been associated with the left inferior frontal gyrus, while semantic fluency is not, 42 suggesting this frontal region may be affected in this PTSD group. Neuroimaging studies have implied that there is dysfunction in the inferior frontal region in PTSD, with reports of decreased frontal lobe volume in PTSD relative to comparison subjects 43 and decreased activation in functional imaging studies in Broca’s area during trauma evoked memories in PTSD patients. 44 Delineation of the anatomic regions and pathologic changes associated with the cognitive dysfunction in PTSD should provide further insights into the pathogenesis of these deficits.
The trend toward significance of recognition memory for faces is noted here only because of several previous reports of medial temporal structural abnormalities with PTSD. Performance on recognition memory for faces has been associated with right temporal lobe pathology, 34 and PTSD neuroimaging studies have shown asymmetrically smaller right hippocampi 45 —this finding is one of the clearest links between function and pathology in PTSD. It is notable that this potential difference is present in the PTSD + comorbid psychiatric pathology group and not in the PTSD only group, suggesting that PTSD alone may not be a sufficient condition for this level of relative impairment.
There ostensibly appear to be two groups of cognitive markers associated with PTSD in this population—those premorbid markers unrelated to the trauma (relatively high IQ) and those associated primarily with developing PTSD. Of those cognitive dysfunctions that appear to be associated with developing PTSD, the most robust quantitative marker of trauma correlated with them is percent weight loss during POW confinement, which in these subjects is thought to represent an individual’s response to the stress of imprisonment. 46 The focal cognitive dysfunctions associated with PTSD in this population (psychomotor speed with Symbol Digit, phonemic fluency as measured by Controlled Oral Word Association, and recognition memory for faces assessed with the Recognition Memory Test) were significantly correlated with percent weight loss, further suggesting that these are unlikely to be premorbid risk factors for developing PTSD.
The cognitive deficits reported here have been reported previously with PTSD; however, not all of these studies specified whether the patients had PTSD alone or had other psychiatric comorbidities. Our findings suggest that PTSD alone may not be sufficient to result in some of these deficits but that they occur only when the subjects have PTSD and comorbid psychiatric diseases.
These findings may also have significant implications for aging, specifically the finding of higher IQ levels in those who do not develop PTSD. Relatively higher IQ status has been suggestive to be protective against developing progressive cognitive decline with aging, 47 including developing dementia. 48 , 49 One study has directly assessed aging in PTSD among Holocaust survivors and found that those with PTSD had a lower IQ than those without PTSD and elderly control subjects not exposed to the Holocaust. More important, explicit recall was impaired for the PTSD group, with 36% (N=31) of these patients impaired to a level of clinical significance. Older age was significantly associated with poorer memory performance in the PTSD groups, but not in the two control groups, with accelerated memory decline one of the more likely explanations for the significantly greater association of older age with poorer explicit memory in the PTSD groups. 50 These results from Holocaust survivors and the results of this study would suggest that older survivors with PTSD (particularly with relatively lower IQ and prevalence of comorbid psychiatric illnesses) may be at risk for developing progression of cognitive dysfunction with aging. The most likely group would be those with existing memory deficits. The present group of Vietnam-era veterans with PTSD should be monitored for progression of accelerated cognitive decline with aging. In addition, there have been reports of an increase in re-experiencing PTSD symptoms concurrent with the development of dementia in several patients (i.e., via loss of frontal lobe suppression of these symptoms as aging diminishes the effectiveness of frontal lobe function). 51 Further studies assessing these factors prospectively in the aging Vietnam-era veterans with PTSD are indicated to better address this issue in terms of preventative, diagnostic, and treatment options. The present study did not address these factors in that none of the subjects reported any subjective complaints nor were any showing signs of dementia. We acknowledge that this POW group is select and represents those individuals who survived until later life without cognitive complaints. These individuals were chosen as they represent a group unlikely to have cognitive difficulties and thus provide the clearest evidence if cognitive deficits existed with PTSD.
There are several limitations to the present study. The number of subjects is relatively small and had a higher education level than the general population. They are also a selective group of POWs who lived into their eighth decade and are being assessed years after the traumatic event occurred. While these issues could limit the ability to generalize the findings, this population without a significant history of alcohol and/or substance abuse and including a group of veterans with PTSD only render this study population uniquely informative.
Overall, the present study provides a well-defined cohort with documented trauma and minimal comorbidities to aid in delineating cognitive dysfunction that is most likely associated with PTSD itself. Our findings suggest that higher IQ is a premorbid protective factor providing resilience from developing PTSD after a traumatic experience. Compared to those POWs who experienced similar traumas and did not develop PTSD, those who developed PTSD (with either psychiatric comorbidities or PTSD alone) had deficits in a selective test of frontal lobe function (Trails B) and visual scanning and psychomotor speed (Symbol Digit). Those with PTSD and psychiatric comorbidities had additional deficits in another selective test of frontal lobe function (phonemic fluency) and a trend toward a decrement in recognition memory for faces. These findings have important implications for other patient populations (e.g., child physical abuse, sexual abuse, involvement in a life threatening accident, natural disaster, or witness of another being badly injured or killed) with a high prevalence of PTSD.
1 . Kessler RC, Sonnega A, Bromet E, et al: Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry 1995; 52:1048–1060Google Scholar
2 . Brandes D, Ben-Schachar G, Gilboa A, et al: PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res 2002; 110:231–238Google Scholar
3 . Bremner JD, Scott TM, Delaney RC, et al: Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 1993; 150:1015–1019Google Scholar
4 . Koenen KC, Driver KL, Oscar-Berman M, et al: Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn 2001; 45:64–78Google Scholar
5 . Moriyama Y, Mimura M, Kato M, et al: Executive dysfunction and clinical outcome in chronic alcoholics. Alcohol Clin Exp Res 2002; 26:1239–1244Google Scholar
6 . Ratti MT, Bo P, Giardini A, et al: Chronic alcoholism and the frontal lobe: which executive functions are impaired? Acta Neurol Scand 2002; 105:276–281Google Scholar
7 . Sullivan EV, Fama R, Rosenbloom MJ, et al: A profile of neuropsychological deficits in alcoholic women. Neuropsychology 2002; 16:74–83Google Scholar
8 . Bremner JD, Southwick SM, Darnell A, et al: Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry 1996; 153:369–375Google Scholar
9 . Jacobsen LK, Southwick SM, Kosten TR: Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry 2001; 158:1184–1190Google Scholar
10 . Block RI, Erwin WJ, Ghoneim MM: Chronic drug use and cognitive impairments. Pharmacol Biochem Behav 2002; 73:491–504Google Scholar
11 . Spikman JM, Deelman BG, van Zomeren AH: Executive functioning, attention and frontal lesions in patients with chronic CHI. J Clin Exp Neuropsychol 2000; 22:325–338Google Scholar
12 . Wallesch CW, Curio N, Kutz S, et al: Outcome after mild-to-moderate blunt head injury: effects of focal lesions and diffuse axonal injury. Brain Inj 2001; 15:401–412Google Scholar
13 . Polo MD, Newton P, Rogers D, et al: ERPs and behavioural indices of long-term preattentive and attentive deficits after closed head injury. Neuropsychologia 2002; 40:2350–2359Google Scholar
14 . Chemtob CM, Muraoka MY, Wu-Holt P, et al: Head injury and combat-related posttraumatic stress disorder. J Nerv Ment Dis 1998; 186:701–708Google Scholar
15 . Brady KT, Killeen TK, Brewerton T, et al: Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry 2000; 61:22–32Google Scholar
16 . Porter RJ, Gallagher P, Thompson JM, et al: Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 2003; 182:214–220Google Scholar
17 . Orsillo SM, Weathers FW, Litz BT, et al: Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. J Nerv Ment Dis 1996; 184:307–313Google Scholar
18 . Vasterling JJ, Duke LM, Brailey K, et al: Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology 2002; 16:5–14Google Scholar
19 . Sutker PB, Galina ZH, West JA, et al: Trauma-induced weight loss and cognitive deficits among former prisoners of war. J Consult Clin Psychol 1990; 58:323–328Google Scholar
20 . Weathers F, Keane TM, Davidson JR: Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001; 13:132–156Google Scholar
21 . Fleming M, Difede J: Effects of varying scoring rules of the Clinician Administered PTSD Scale (CAPS) for the diagnosis of PTSD after acute brain injury. J Traum Stress 1999; 12:535–542Google Scholar
22 . First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
23 . Folstein MF, Folstein SE, McHugh PR: “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
24 . Blair JR, Spreen O: predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol 1989; 3:129–136Google Scholar
25 . Goodglass H, Kaplan E, Weintraub S: Boston Naming Test. Philadelphia, Lea and Febiger, 1983Google Scholar
26 . Goodglass H, Kaplan E: The Assessment of Aphasia and Related Disorders, 2nd ed. Philadelphia, Lea and Febiger, 1983Google Scholar
27 . Spreen O, Benton A: Neurosensory Center Comprehensive Examination for Aphasia. Victoria, BC, University of Victoria, 1969Google Scholar
28 . Benton AL, Hamsher KD: Multilingual Aphasia Examination. Iowa City, University of Iowa, 1976Google Scholar
29 . U.S. War Department: Manual of directions and scoring, in Army Individualized Test. Washington, DC, US War Department, Adjutant General’s Office, 1944Google Scholar
30 . Reitan RM: Manual for administration of neuropsychological test batteries for adults and children. Tucson, Ariz, Neuropsychology Press, 1979Google Scholar
31 . Wechsler D: Wechsler Adult Intelligence Scale-III. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
32 . Rey A: L’examen Clinique en Psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
33 . Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corporation, 1987Google Scholar
34 . Warrington EK: Recognition Memory Test. London, Nfer-Nelson, 1984Google Scholar
35 . Benton AL, Sivan AB, Hamsher KD, et al: Contributions to Neuropsychological Assessment. New York, Oxford University Press, 1983Google Scholar
36 . Smith A: Symbol Digit Modalities Test. Los Angeles, Calif, Western Psychological Services, 1982Google Scholar
37 . Brink TL, Swartz M, Woodbury M, et al: Depressive symptoms and depressive diagnosis in a community population. Arch Gen Psychiatry 1982; 45:1078–1084Google Scholar
38 . Yesavage JA, Brink TL, Rose TL, et al: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49Google Scholar
39 . Shum DHK, McFarland KA, Bain JD: Construct validity of eight tests of attention: comparison of normal and closed head injury samples. Clin Psychologist 1990; 1:151–162Google Scholar
40 . Segalowitz SJ, Unsal A, Dywan J: CNS evidence for the distinctiveness of frontal and posterior neural processes in a traumatic brain-injured population. J Clin Exp Neuropsychol 1992; 14:545–565Google Scholar
41 . Fennema-Notestine C, Stein MB, Kennedy CM, et al: Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52:1089–1101Google Scholar
42 . Paulesu E, Goldacre B, Scifo P, et al: Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 1997; 8:2011–2017Google Scholar
43 . Carrion VG, Weems CF, Eliez S, et al: Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001; 50:943–951Google Scholar
44 . Hull AM: Neuroimaging findings in post-traumatic stress disorder: systematic review. Br J Psychiatry 2002; 181:102–110Google Scholar
45 . Bremner JD, Randall P, Scott TM, et al: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Google Scholar
46 . Thygesen P, Hermann K, Willanger R: Concentration camp survivors in Denmark persecution, disease, disability, compensation: a 23-year follow-up. A survey of the long-term effects of severe environmental stress. Dan Med Bull 1970; 17:65–108Google Scholar
47 . Cervilla JA, Prince M, Joels S, et al: Long-term predictors of cognitive outcome in a cohort of older people with hypertension. Br J Psychiatry 2000; 177:66–71Google Scholar
48 . Schmand B, Smit JH, Geerlings MI, et al: The effects of intelligence and education on the development of dementia: a test of the brain reserve hypothesis. Psychol Med 1997; 27:1337–1344Google Scholar
49 . Alexander GE, Furey ML, Grady CL, et al: Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry 1997; 154:165–172Google Scholar
50 . Gollier J, Yehuda R, Lupien SJ, et al: Memory performance in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry 2002; 159:1682–1688Google Scholar
51 . Mittal D, Torres R, Abashidze A, et al: Worsening of post-traumatic stress disorder symptoms with cognitive decline: case series. J Geriatr Psychiatry Neurol 2001; 14:17–20Google Scholar