MK 801: A Possible Neuroprotective Agent by Poststroke Depression?
To the Editor: Stroke is the third leading cause of death and adult morbidity in developed countries. 1 Many deleterious cellular pathways have been proposed to explain the molecular pathogenesis of this clinically devastating disease. 2 Interest in the role of neurotransmitters in the pathogenesis of ischemic stroke resulted in studies establishing that the release of glutamate and its excitotoxic actions through the N -methyl- D -aspartic acid (NMDA) receptors are significant in the development of ischemic neuronal damage. 2
It is widely known that the occurrence of poststroke mood disorders, especially depression, is one of the most frequent complications of stroke. 3 It affects approximately 20% to 40% of all patients and the definitive treatment includes various antidepressant agents. 3 Interestingly, the evidence regarding antidepressant activity of NMDA receptor antagonists (especially MK 801) is rapidly replicating. 4 , 5 In light of these findings, we wanted to evaluate whether this novel antidepressant agent also has a neuroprotective effect by cerebral ischemia.
To examine this matter, we evaluated the neuroprotective effect of MK 801 (dizocilpine), a noncompetitive NMDA receptor antagonist, after transient focal cerebral ischemia, a relevant model for the thrombolyzed stroke in humans.
Anesthetized Wistar rats (330–370 g) were submitted to transient thread occlusion of the middle cerebral artery using an intraluminal filament technique. 6 The rectal temperature was maintained between 36.5°C and 37.0°C using a feedback-controlled heating system. The cerebral blood flow, measured by laser Doppler flowmetry, was reduced to ∼15% of preischemic control levels immediately after thread insertion in all animal groups. MK 801 or vehicle was applied intraperitoneally (3 mg/kg) just after transient ischemia. Twenty-four hours after reperfusion, the area of infarction was measured by toluidine blue staining. This study was interesting with regard to poststroke depression by which a mood stabilizer with neuroprotective properties would be preferable.
MK 801 showed a significant neuroprotection (p<0.05, analysis of variance followed by least significant difference tests) after transient focal cerebral ischemia (73.12±23.2 versus 23.12+21.1) ( Figure 1 ). In summary, this study indicates that even with a serious brain injury, MK 801 could exert a significant neuroprotective effect. However, further experiments to evaluate the long-term clinical reflections of such neuroprotective effects of MK 801 in poststroke depression patients via MRI and spectroscopy studies, would be logical future steps in the field of psychiatric research.
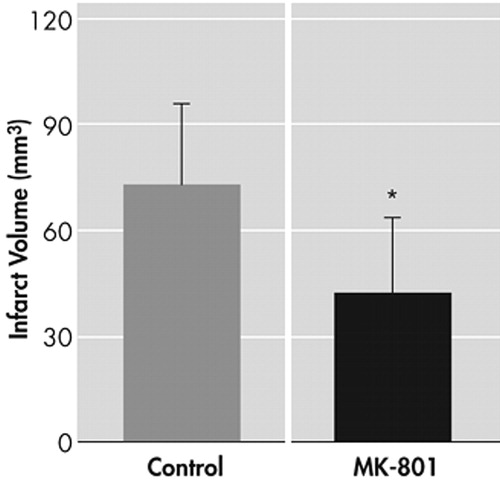
Data are given as means±SD
Animals treated with MK 801 (3 mg/kg) led to a decrease in infarct size.
*Significantly different from vehicle-treated control animals (p≤0.05).
The first two authors contributed equally to this work.
1 . Rothwell PM: The high cost of nonfunding stroke research: a comparison with heart disease and cancer. Lancet 2001; 357:1612–1616.Google Scholar
2 . Dirnagl U, Iadecola C, Moskowitz MA: Pathobiology of ischemic stroke: an integrated view. Trends Neurosci 1999; 22:391–397Google Scholar
3 . Whyte EM, Mulsant BH: Poststroke depression: epidemiology, pathophysiology and biological treatment. Biol Psychiatry 2002; 52:253–264Google Scholar
4 . Zomkowski AD, Hammes L, Lin J, et al: Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport 2002; 25:387–391Google Scholar
5 . Chaturvedi HK, Bapna JS, Chandra D: Effect of fluvoxamine and N -methyl- D -aspartate receptor antagonists on shock-induced depression in mice. Indian J Physiol Pharmacol 2001; 45:199–207. Google Scholar
6 . Koizumi J, Yoshida Y, Nakazawa T, et al: Experimental studies of ischemic brain edema. Part I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke 1986; 8:1–8 (Japanese)Google Scholar