Neural Correlates of Automatic and Controlled Auditory Processing in Schizophrenia
Given the deficits in automatic and controlled auditory information processing in schizophrenia, it is important to compare the neural systems associated with these two aspects of auditory information processing and their changes with illness duration. Electrophysiological indices show differences between automatic and controlled processing that are further modulated by illness duration, 12 but have not been assessed with functional magnetic resonance imaging (fMRI). The primary goal of this study was to investigate differences in brain function associated with schizophrenia during controlled and automatic auditory processing. A secondary goal was to examine relations between functional brain differences and illness duration. We hypothesized that patients would show impaired prefrontal, superior temporal, and striato-thalamic responses during auditory information processing relative to participants in the comparison group. We further hypothesized that differences in regional brain activation between automatic and controlled auditory processing would be more pronounced in the comparison group relative to the patient group. This prediction was based on evidence that consistently shows that patients with schizophrenia produce lower overall task-related neural activity (i.e., compared to baseline) as well as reduced condition effects across many cognitive domains. 3 , 6 , 13 Furthermore, we predicted that frontal, superior temporal, and striato-thalamic function would deteriorate with illness duration. We employed an auditory oddball task because it elicits activation of frontotemporal regions in healthy comparison subjects, 14 it requires top-down organization and integration of auditory information, 14 , 15 and patients with schizophrenia exhibit impaired task-associated neural function. 4 , 12 , 16
METHOD
Participants
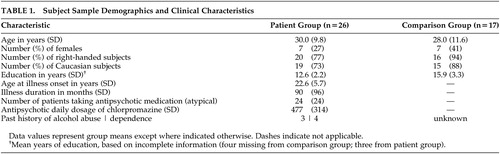
The study participants included 26 patients with schizophrenia and 17 neuro-psychiatrically healthy comparison subjects. Participant groups were matched for age (t=0.61, df=41, p=0.27), gender (χ 2 =0.95, df=1, p=0.33), handedness (χ 2 =2.2, df=1, p=0.14), 17 and race (African-American or European-American) (χ 2 =1.4, df=1, p=0.23). Years of education was calculated based on missing data from four comparison subjects and three schizophrenia patients and was greater in the comparison group (t=3.4, df=35, p<0.001). Demographic and clinical characteristics are summarized in Table 1 .
 |
Diagnoses were confirmed by the Structured Clinical Interview for DSM-IV (SCID) and rated using the Symptom Onset in Schizophrenia inventory. 18 Patients experiencing acute psychotic symptoms, active substance use disorders, or pregnancy were excluded. Patients were recruited from inpatient and outpatient facilities affiliated with the University of North Carolina at Chapel Hill. Comparison subjects with a history of psychiatric illness or a first-degree relative with psychotic symptoms were excluded. All participants received a verbal explanation of the study and provided written informed consent for procedures approved by the institutional review boards at UNC-Chapel Hill and Duke University Medical Center.
Stimuli and Tasks
Participants performed two tasks consecutively in a single session. The first was an automatic auditory detection task in which participants were presented with task-irrelevant frequent standard tones and infrequent pitch-deviant tones (unattended auditory deviants) while performing a visual discrimination task that required a forced choice response between infrequent targets and frequent nontargets. The second was a controlled auditory processing task during which participants responded with a unique button press to infrequently occurring pitch-deviant tones (attended auditory target) and an alternate button press to frequently occurring standard tones. At the same time, participants passively viewed nontarget stimuli (squares) to facilitate objective comparison with the first task. Visual and auditory information were presented synchronously for both tasks. Although different from the traditional oddball paradigm which requires a response only to rare events, the forced-choice paradigm enables examination of reaction time data for all trials and has previously been shown to elicit a homologous pattern of activation to rare events as the target detection version. 19
Subjects completed the unattended auditory deviant (UAD) runs prior to the attended auditory target (AAT) runs in order to minimize possible priming of the UAD tones due to their role in the AAT condition. Exposure to AAT trials prior to the UAD trials could lead to stronger orienting to the UAD stimuli and therefore contaminate a task that was designed to measure pure automatic processing. This kind of orienting and contamination is likely to be greater in comparison subjects than in patients, as suggested by the latent inhibition literature. 20
For both tasks, frequently occurring (90%) standard tones (1000 Hz) and infrequently occurring (10%) deviant tones (1064 Hz) tones of 68 ms duration were delivered through headphones (Resonance Technology Inc., Northridge, Calif.) at ∼85 dB with a stimulus onset asynchrony of 1500 ms. Button assignment for target and nontarget stimuli was counterbalanced across participants. Right-handed behavioral responses on a fiber-optic response box were time-locked to fMRI data acquisition for 42 UAD trials over seven runs (∼5 minutes/run; 200 image volumes), followed by 42 AAT trials over three runs (∼4 minutes/run; 160 image volumes). Relevant trials were separated by a random interval of 15 to 18 seconds to enable full recovery of the hemodynamic response to baseline and facilitate event-related fMRI analysis. The UAD trials were spaced further apart to accommodate for trials from the visual discrimination task resulting in a greater number of image volumes corresponding to the UAD trials (200×7 runs) than AAT trials (160×3 runs).
Acquisition of MRI Data
Images were acquired on a 1.5 Tesla GE Signa scanner with a birdcage type standard quadrature head coil and an advanced nuclear magnetic resonance echo planar system. The participant’s head was positioned along the canthomeatal line and immobilized using a vacuum cushion and a forehead strap. High-resolution T1 weighted anatomical images (3D SPGR, TR =22 ms, TE=5 ms, flip angle =20°, FOV =24 cm, voxel =0.9375×0.9375×1.5 mm, 256×256 voxels, 24 images), two-dimensional spin echo sequence (TR=4000ms, TE=20, flip angle=90°, FOV=24 cm, 1.5 mm slice thickness), and 16 contiguous axial coplanar anatomical images parallel to the anterior commissure-posterior commissure plane coincident with 16 functional slices using a gradient echo planar sequence (TR=1500ms, TE=40ms, flip angle=90°, NEX=1, voxel=3.75×3.75×5 mm, imaging matrix 64×64 voxels) were acquired.
Functional Image Analysis
Our preliminary analysis method involved the computation of random-effects activation and contrast maps, which was followed up by a confirmatory region of interest analysis method. This approach has been used in many previous studies. 3 , 21 Head motion was detected by center of mass measurements in three orthogonal planes. On the basis of these measurements, runs containing movement greater than 1 mm from baseline were rejected, resulting in the elimination of single runs in one comparison subject and four patients (<1% of the total data). Image preprocessing (slice timing correction, motion correction, coregistration, normalization, smoothing) was performed using SPM99. Post-processing and quantitative analysis were performed using custom Matlab software (Duke-UNC BIAC, Durham, N.C.) using the procedures described.
Random Effects Analysis
The preliminary analysis consisted of a random-effects assessment of the differences among the conditions and groups based on the event-related hemodynamic response. In a whole-brain voxel-based approach, individual participant t-maps were generated for each target event (AAT and UAD trials) by identifying voxels in which the average activity correlated with an empirically derived hemodynamic response template. First, epochs of 15 consecutive image volumes were extracted from the raw signal coincident with each target event (an AAT or UAD). Each epoch consisted of five image volumes preceding the target event (at −7.5, −6, −4.5, −3, and −1.5 seconds), nine image volumes following the target event (at 1.5, 3, 4.5, 6, 7.5, 9, 10.5, 12, and 13.5 seconds) and the image volume coincident with the target event (at 0 seconds). Epochs were averaged separately for the AAT and UAD conditions. Second, the BOLD signal time series for each voxel was correlated with an empirical reference waveform, and t-statistics were calculated for the correlation coefficients for each voxel. A significance threshold of p<0.0002 (uncorrected) was used to determine active voxels. 22 The empirical waveform was obtained based on resampling a hypersampled waveform obtained according to published procedures. 23 This procedure provided a whole-brain t-map in Montreal Neurological Institute space for each condition. 3 The individual t-maps created in the preceding step were then subjected to a random-effects analysis that assessed the significance of differences between conditions across participants, as well as differences between participant groups for UAD and AAT conditions, respectively. Random-effects analyses generated contrast maps to show significant differences in activation between conditions (AAD versus UAD) and between groups (patient versus comparison). Contrast maps were masked with active voxels from the original t-maps and displayed on a template anatomical image.
Regions of Interest Analysis
Specific hypotheses were tested using a region of interest-based analysis. 3 , 21 Six a priori anatomical regions of interest were defined (see Figure 1 ): anterior cingulate gyrus (ACG), inferior frontal gyrus (IFG), middle frontal gyrus (MFG), superior temporal gyrus (STG), basal ganglia, and thalamus. These regions of interest were manually traced on individual participants’ high-resolution coplanar images using anatomical landmarks by a single rater blind to diagnosis, according to procedures described by the Laboratory of Neuro Imaging (University of California, Los Angeles, http://www.loni.ucla.edu/). The selection of regions that were hypothesized a priori on the basis of the literature permitted us to relax the requirement for multiple comparison correction. Voxels with t>1.96 (p<0.05, uncorrected) were accepted as activated to compute the number of active voxels in the region of interest, then divided by the total number of voxels in the region of interest to obtain the percentage of activated voxels. The average percentage signal change at each time point in the event epoch was calculated within each region of interest. Analysis of the UAD condition was performed on all trials. Analysis of the AAT condition was performed first for all trials, then again for correct trials only.
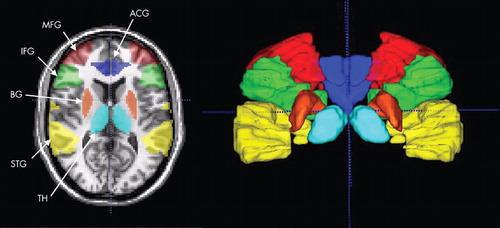
Regions are color coded: dark blue=anterior cingulate gyrus (ATG), green=inferior frontal gyrus (IFG), red=middle frontal gyrus (middle frontal gyrus), yellow=superior temporal gyrus (STG), orange=basal ganglia (BG), and light blue=thalamus (TH).
Correlation Analysis Between Activation and Clinical-Behavioral Measures
We investigated the relationship between brain activation (percentage of activated voxels and percentage signal change) for the six regions of interest and clinical illness duration, percent hits on the AAT task, and antipsychotic dosing (chlorpromazine equivalents).
RESULTS
Behavioral Performance
In the attended auditory target (AAT) controlled processing task, patients made fewer hits, detection or correct responses (mean±SE of means; 57.5±5.0%), than the comparison subjects (76.6±5.6%; t=2.5, df=41, p<0.01), but more misses, or false negatives (36.6±5.2%), than comparison subjects (23.5±4.5%; t=1.9, df=41, p<0.05). There were no significant group differences for nonresponses (t=1.1, df=41, p=0.13; patients=5.8±2.3%; comparison=2.3±1.5%), for latency on correct trials (t=1.7, df=41, p=0.43; patients=516±23 msec; comparison=510±28 msec), or for incorrect trials (t=1.1, df=41, p=0.13; patients=414±60 msec; comparison =320±21 msec). During the unattended auditory deviants (UAD) automatic processing task, subjects performed a visual discrimination task. Patients again made fewer hits (54±4%) than comparison subjects (74±4%; t=3.1, df=41, p<0.005). We tested for the possibility of order effects (e.g., habituation) given that the UAD trials always preceded AAT trials. Performance data from the visual discrimination task in runs 1 through 7 showed no differential decline in performance between patients and comparison subjects (F=0.4, df=1, 290, p=0.53).
Random-Effects Analyses
Contrast maps show results of random-effects analyses between conditions ( Figure 2 ) and participant groups ( Figure 3 ). Overall, patients showed lower activation than comparison subjects for both the AAT and UAD conditions. Patients showed lower activation in the UAD condition in anterior and posterior left superior temporal gyrus, as well as in the middle frontal gyrus and inferior frontal gyrus bilaterally. Patients showed lower activation for the AAT condition in dorsal anterior cingulate gyrus, and bilaterally in middle frontal gyrus, inferior frontal gyrus (L>R), insula, basal ganglia, and thalamus. There were no areas in which activation in the patient group was greater than the comparison group.
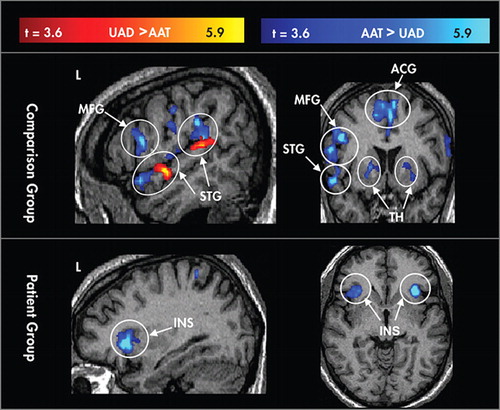
Areas where the contrast for attended auditory target (AAT) > unattended auditory deviant (UAD) are coded in blue, and areas where UAD>AAT are coded in red. Controlled processing showed greater activation in both groups in the bilateral insula (INS). The comparison group showed additional areas with greater activation for controlled processing including the left middle frontal gyrus (MFG), thalamus (TH), dorsal anterior cingulate gyrus (ACG), and anterior and posterior aspects of the left superior temporal gyrus (STG).
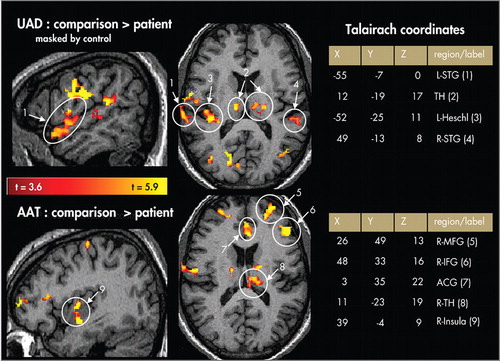
Upper panels=unattended auditory deviant (UAD) condition in superior temporal gyrus (STG), Heschl’s gyrus (primary auditory cortex), and thalamus. Lower panels=random-effects contrast maps showing comparison > patient activation for the attended auditory target (AAT) condition in middle frontal gyrus (MFG), inferior frontal gyrus (IFG), anterior cingulate gyrus (ACG), thalamus (TH), and insula.
Controlled auditory processing showed generally greater activation relative to automatic processing (AAT>UAD) in both patients and comparison subjects with a few exceptions. Controlled processing elicited greater activation than automatic processing in both groups in the bilateral insula. Controlled processing also elicited greater activation for comparison subjects in the left middle frontal gyrus, thalamus, dorsal anterior cingulate gyrus, and anterior and posterior aspects of the left superior temporal gyrus. By contrast, automatic processing elicited greater activation than controlled processing in a focal area of the rostral anterior cingulate gyrus for both participant groups, and for the comparison group only, in the anterior and posterior portions of the left superior temporal gyrus (see Figure 2 ).
Regions of Interest Analyses
The percentage of activated voxels within each anatomical region of interest was analyzed as the dependent variable in an omnibus analysis of variance (ANOVA) with factors of group (patient, comparison), region (ACG, IFG, MFG, STG, basal ganglia, thalamus), condition (AAT, UAD), and hemisphere (Left, Right). This analysis resulted in main effects for group (F=7.8, df=1, 41, p<0.01), region (F=6.5, df=5, 205, p<0.0001], condition (F=40.3, df=1, 41, p<0.0001), and hemisphere (F=21.1, df=1, 41, p<0.0001), as well as interaction effects for region×group (F=2.3, df=5, 205, p<0.05) and region×condition (F=5.1, df=5, 205, p<0.005), and a trend level interaction for region×condition×group (F=2.0, df=5, 205, p<0.08), but no effect for hemisphere×group (F=0.04, df=1, 41, p=0.85). As seen in Figure 4 , post hoc analyses showed greater overall extent of activation in comparison subjects than patients (main effect of group) and greater activation to the AAT condition than UAD condition (main effect of condition). Post hoc analyses of the UAD condition revealed that the patient group had a smaller extent of activation in the superior temporal gyrus (t=4.21, df=41, p<0.05), thalamus (t=5.6, df=41, p<0.05), and middle frontal gyrus (t=4.6, df=41, p<0.05). Post hoc analysis of the AAT condition showed smaller extent of activation in the patient group in inferior frontal gyrus (t=1.7, df=41, p<0.05), basal ganglia (t=2.1, df=41, p<0.02), and thalamus (t=2.5, df=41, p<0.01), and trending smaller in middle frontal gyrus (t=1.6, df=41, p<0.06).
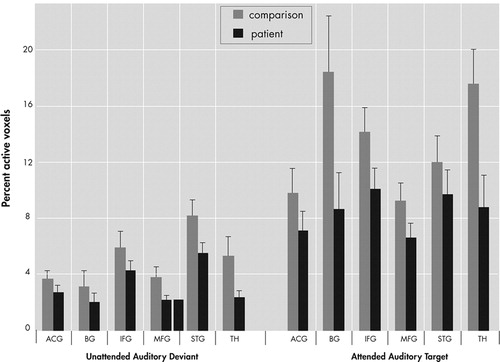
Patients showed lower overall extent of activation than comparison subjects (main effect of group). Activation for the UAD condition was lower than for the AAT condition (main effect of condition). ACG=anterior cingulate gyrus; IFG=inferior frontal gyrus; MFG=middle frontal gyrus; STG=superior temporal gyrus; BG=basal ganglia; TH=thalamus
This analysis was repeated using percent signal change as the dependent variable and results were generally consistent with the preceding analysis. An omnibus ANOVA using PSC as the dependent variable revealed main effects for group (F=5.46, df=1, 41, p<0.03), region (F=22.4, df=5, 205, p<0.0001), and condition (F=17.5, df=1, 41, p<0.0001), and an interaction of region×condition (F=2.5, df=5, 205, p<0.05); there was no main effect for hemisphere (F=1.4, df=1, 41, p=0.25) and no interaction effects for region×group (F=0.73, df=5, 205, p<0.6), condition×group (F=0.11, df=1, 41, p<0.75), hemisphere×group (F=0.91, df=1, 41, p=0.35) or region×condition×group (F=0.69, df=5, 205, p<0.63). Post hoc analyses showed greater overall intensity of activation in comparison subjects relative to patients and in the AAT condition relative to the UAD condition. Post hoc analysis showed lower AAT activation in the patient group in inferior frontal gyrus (t=2.1, df=41, p<0.05) and thalamus (t=2.3, df=41, p<0.02), and for the UAD condition in thalamus (t=2.7, df=41, p<0.01) and marginally lower in the left superior temporal gyrus (t=1.6, df=41, p=0.06).
As the two groups differed in their performance accuracy in the AAT condition, we reanalyzed the data from this condition using only trials with correct responses (the UAD condition was not reanalyzed since no behavioral responses were involved). This analysis resulted in a marginal main effect for group (F=3.6, df=1, 41, p<0.07), a significant main effect for hemisphere (F=7.6, df=1, 41, p<0.01), and for region (F=6.7, df=5, 205, p<0.0001), as well as significant interaction effects for region×group (F=2.4, df=5, 205, p<0.04), region×hemisphere (F=7.1, df=5, 205, p<0.0001), and region×hemisphere×group (F=4.2, df=5, 205, p<0.005). These effects parallel those from the analysis of all trials. Post hoc analyses showed a smaller spatial extent of activation in patients than comparison subjects in the right inferior frontal gyrus (t=2.1, df=41, p<0.01), left basal ganglia (t=2.6, df=41, p<0.01), and the left anterior cingulate gyrus (t=1.8, df=41, p<0.05).
A post hoc analysis of the middle frontal gyrus and superior temporal gyrus showed that AAT activation in the middle frontal gyrus was highly correlated with UAD activation in superior temporal gyrus in patients (percentage of activated voxels; r=0.70, p=0.0001), but not in comparison subjects (r=0.12, p=0.65).
Effects of Clinical Measures
There was a negative correlation between illness duration and percentage of activated voxels for the AAT condition in the superior temporal gyrus (r=−0.51, p<0.01). Because age was highly correlated with illness duration (r=0.81, p<0.0001), we also examined whether superior temporal gyrus activation varied with age in comparison subjects; no effect was observed (r=0.08, p<0.77). More modest negative correlations were also found between illness duration and percentage of activated voxels to the AAT condition in the thalamus (r=−0.38, p<0.06) and inferior frontal gyrus (r=−0.35, p<0.08). For percentage signal change, only the superior temporal gyrus (r=−0.38, p<0.06) was significantly correlated with illness duration for the AAT condition. All these measures were uncorrelated with age in comparison subjects. Negative correlations between medication dose and percentage of activated voxels for the AAT condition were noted in the subcortical regions, basal ganglia (r=−0.42, p<0.04) and thalamus (r=−0.38, p=0.06). Activations in frontal and temporal regions were uncorrelated with medication dose.
DISCUSSION
We assessed functional brain differences with BOLD activation during automatic and controlled auditory information processing in participants with schizophrenia and age-matched comparison subjects. During unattended auditory processing, patients showed lower activation than comparison subjects in the middle frontal gyrus and thalamus as well as in the superior temporal gyrus. During controlled auditory processing, patients showed lower activation in the middle frontal gyrus, inferior frontal gyrus, anterior cingulate gyrus, insula, and subcortical regions.
Functional Changes in Schizophrenia With Automatic Processing
Our results demonstrated reduced activation in patients relative to comparison subjects during automatic auditory processing in the right middle frontal gyrus, thalamus, and superior temporal gyrus. This suggests that auditory impairment in schizophrenia extends beyond the superior temporal gyrus and Heschl’s gyrus as shown in earlier fMRI studies, 10 , 24 , 25 and as inferred from event-related potential studies of the auditory mismatch negativity. 26 Impaired thalamic function has been reported in controlled processing, 4 but not in automatic processing. Studies of comparison subjects show that automatic auditory processing occurs primarily at the level of the primary auditory cortex. 15 , 27 However, preattentive change detection involves further interaction between sensory and prefrontal cortices that can facilitate potential behavioral responses by engaging frontal networks in response to sensory inputs. 14 Evidence of a frontal contribution to mismatch negativity that deteriorates in schizophrenia is suggested by event-related potential findings, 28 , 29 but this has not been demonstrated with neuroimaging until now. Thus, our findings show that prefrontal dysfunction in schizophrenia is associated not only with higher cognitive functions, 4 , 11 but with sensory level auditory processing as well. It is possible that the middle frontal gyrus dysfunction we observed with automatic processing may underlie the clinically observed distractibility in schizophrenia and may reflect a porous mechanism for filtering sensory stimuli in noisy environments. 30
Evidence shows that the brain’s response to task-irrelevant sensory changes is strongly influenced by intermodal attentional demands. 31 We have previously published findings from healthy subjects performing a continuous perceptual-motor visual tracking task at two levels of difficulty while simultaneously hearing a series of task-irrelevant standard tone pips and infrequent pitch-deviant tones. Our fMRI results revealed that the unattended pitch-deviant tones strongly activated superior temporal and frontal cortical regions. These activations were significantly modulated by the tracking difficulty of the primary task, 32 but this effect is greater under high load conditions. In this respect, results from the patients in the current study are consistent with the literature describing healthy subjects. In the present study, the primary task is a low load visual discrimination task, perhaps especially so for the comparison group as evidenced by their superior behavioral performance. Thus, the low load visual task may not have been sufficiently demanding to unveil the effect in question in comparison subjects, but it was evident in patients for whom the task difficulty was greater.
Functional Changes in Schizophrenia With Controlled Processing
We observed impaired behavioral performance in patients with schizophrenia during controlled auditory processing, which, consistent with earlier work, was accompanied by lower activation in inferior frontal gyrus, middle frontal gyrus, anterior cingulate gyrus, subcortical structures (basal ganglia, thalamus), and insula. 4 , 6 , 8 Separate region of interest analyses for all trials and for correct trials revealed concordant findings. Patients had lower activation in the inferior frontal gyrus, basal ganglia, and thalamus for both analyses and lower activation in the anterior cingulate gyrus on correct trials. The unique anterior cingulate gyrus finding for the correct trials is plausible given its role in conflict monitoring and error detection 33 and given evidence of its dysfunction in schizophrenia. 34
Recent studies show that ventral limbic structures such as the amygdala, orbitofrontal cortex, and rostral anterior cingulate gyrus are also impaired in schizophrenia during automatic auditory processing. 6 , 8 However, activation of these ventral structures could be attributed to the inclusion of a third novel tone (nontarget) that was not included in the present study. Though we found a relatively focal region of the anterior superior temporal gyrus that showed lower activation in the schizophrenia group in the random effects analysis, the region of interest analysis did not show such a difference. Previous studies are unclear about the superior temporal gyrus, with deficits noted in some studies, 4 , 8 but not others. 6
Comparison of Automatic and Controlled Processing in Schizophrenia
We found that controlled processing generally elicited greater activation relative to automatic processing in both patient and comparison groups. However, for controlled processing, the comparison group showed additional areas with greater activation as compared to the patient group. Despite diminished superior temporal gyrus activity in patients during unattended processing, a corresponding diminution was not detected for attended processing. We have considered two possible explanations for this difference. The first concerns the role of the visual task that was performed concurrently with the unattended auditory task. Here we posit that the greater subjective difficulty of the primary visual task diverted attentional resources in the prefrontal cortex away from automatic processing, which relies, in part, on prefrontal resources. With reduced prefrontal resources now available to automatic processing, an increased load was placed on the normal sensory processing function of the superior temporal gyrus. Thus, superior temporal gyrus dysfunction in the schizophrenia group only became readily apparent under this increased load level. Such effects of primary task complexity on automatic auditory processing have been demonstrated in comparison subjects. 27 The second possible explanation concerns the more distributed processing across heteromodal frontal cortical areas in controlled processing relative to automatic attention processes. Frontal cortical areas may be capable of offloading resource demands from the superior temporal gyrus during controlled processing in a manner that is not possible during automatic processing. Under this low load condition, superior temporal gyrus dysfunction associated with schizophrenia is less likely to manifest than in a high load condition. These theories will require testing using more elaborate experimental paradigms.
Overall, activation differences between automatic and controlled processing were more pronounced in the comparison group than in the patient group. For instance, the comparison group had greater activation in the middle frontal gyrus, dorsal anterior cingulate gyrus, and thalamus for controlled processing; this was not the case in the schizophrenia patient group. Compromised white matter integrity 35 combined with widespread structural 36 and functional brain changes in schizophrenia may limit the ability of the brain to dynamically allocate neural resources according to processing demands. This may lead to schizophrenia patients having a more static allocation strategy of neural resources across varied processing needs. Findings from post hoc analyses support this idea, showing that middle frontal gyrus activation during controlled processing and superior temporal gyrus activation during automatic processing were highly correlated in patients but not in comparison subjects. This may suggest that allocation of neural resources between middle frontal gyrus and superior temporal gyrus is maintained in a relatively fixed relationship regardless of task demands, which is consistent with the idea of schizophrenia as a disconnection syndrome. 2 , 37
Clinical Correlations With Functional Brain Differences
Dysfunction in the superior temporal gyrus correlated with illness duration. Similar trend level relationships were noted in the thalamus and inferior frontal gyrus. As expected, age was highly correlated with illness duration, and therefore superior temporal gyrus dysfunction could also be related to aging. 38 Given the lack of a corresponding effect of age in our comparison group, we attribute the observed superior temporal gyrus dysfunction primarily to illness duration. These results are consistent with earlier findings of P300 prolongation and reduction with illness duration. 39 , 40 Early age of illness onset is also associated with P300 amplitude reduction, 41 greater illness severity, 42 neuropsychological deficits, and structural brain abnormalities. 43 The P300 in the auditory oddball likely comprises both P300a and P300b components, and converging fronto-temporal-parietal and cortico-limbic generators, 44 which are implicated in the neuropathology of schizophrenia. 45
Strengths and Limitations
Most patients in the schizophrenia patient group were being treated with antipsychotic medication. Dysfunction in the basal ganglia and thalamus was correlated with medication dose, but this relationship was not found in frontal or superior temporal gyrus activation. Research using event-related potentials indicates that changes in neural activity during auditory oddball detection in schizophrenia are at least partially independent of medication effects. 46 Our patient group was significantly less accurate than comparison subjects and the group differences in activation measures were congruent with impaired task performance. We found that patients had lower overall activation when considering only correct responses for controlled processing. Moreover, activation differences in automatic processing were independent of behavioral performance. The schizophrenia and comparison groups had disparate educational levels, but this lack of educational progress in the schizophrenia group might be expected based on the age of illness onset. Smoking history of participants was not collected and would be important given the prevalence of smoking in patients with schizophrenia. However, recent evidence now refutes 47 earlier reports that nicotine alters cerebral blood flow. 48 Finally, the attended and unattended task could have been better equated in several respects: by using the same visual presentation for both conditions, circles and squares could have been presented during the attended auditory task, rather than just squares alone. Additionally, target events in the UAD condition were temporally further apart than in the AAT condition.
Previous functional imaging studies in this population have been limited to either automatic or controlled processing. The within-subject design that considers both automatic and controlled processing is a strength of the present study. Previous functional neuroimaging studies in healthy subjects have localized neural activity associated with automatic auditory deviance detection to both superior temporal and prefrontal areas. Studies of automatic processing in patients with schizophrenia show functional brain changes in the superior temporal lobe. Thus, another strength is that we demonstrate that dysfunction in schizophrenia during automatic processing extends beyond the temporal lobe into prefrontal and subcortical structures.
1 . Javitt DC, Doneshka P, Grochowski S, et al: Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry 1995; 52:550–558Google Scholar
2 . Meyer-Lindenberg A, Poline JB, Kohn PD, et al: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Google Scholar
3 . Morey R, Inan S, Perkins D, et al: Imaging frontostriatal function in ultra high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry 2005; 62:254–262Google Scholar
4 . Kiehl KA, Liddle PF: An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophrenia Res 2001; 48:159–171Google Scholar
5 . O’Leary DS, Andreasen NC, Hurtig RR, et al: Auditory attentional deficits in patients with schizophrenia: a positron emission tomography study. Arch Gen Psychiatry 1996; 53:633–641Google Scholar
6 . Liddle PF, Laurens KR, Kiehl KA, et al: Abnormal function of the brain system supporting motivated attention in medicated patients with schizophrenia: an fMRI study. Psychol Med 2006; 36:1097–1108Google Scholar
7 . Laurens KR, Kiehl KA, Ngan ET, et al: Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophrenia Res 2005; 75:159–171Google Scholar
8 . Kiehl KA, Stevens MC, Celone K, et al: Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol Psychiatry 2005; 57:1029–1040Google Scholar
9 . Wible CG, Kubicki M, Yoo SS, et al: A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry 2001; 158:938–943Google Scholar
10 . Kreitschmann-Andermahr I, Rosburg T, Meier T, et al: Impaired sensory processing in male patients with schizophrenia: a magnetoencephalographic study of auditory mismatch detection. Schizophrenia Res 1999; 35:121–129Google Scholar
11 . Andreasen NC, O’Leary DS, Flaum M, et al: Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 1997; 349:1730–1734Google Scholar
12 . Umbricht DSG, Bates JA, Lieberman JA, et al: Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset, and chronic schizophrenia. Biol Psychiatry 2006; 59:762–772Google Scholar
13 . Tan H-Y, Sust S, Buckholtz JW, et al: Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry 2006; 163:1969–1977Google Scholar
14 . Opitz B, Rinne T, Mecklinger A, et al: Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage 2002; 15:167–174Google Scholar
15 . Mitchell TV, Morey RA, Inan S, et al: Functional magnetic resonance imaging measure of automatic and controlled auditory processing. Neuroreport 2005; 16:457–461Google Scholar
16 . van der Stelt O, Frye J, Lieberman JA, et al: Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry 2004; 61:237–248Google Scholar
17 . Oldfield RC: The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 1971; 9:97–113Google Scholar
18 . Perkins DO, Leserman J, Jarskog LF, et al: Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) inventory. Schizophrenia Res 2000; 44:1–10Google Scholar
19 . Braver TS, Barch DM, Gray JR, et al: Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex 2001; 11:825–836Google Scholar
20 . Lubow RE, Gewirtz JC: Latent inhibition in humans: data, theory, and implications for schizophrenia. Psychol Bull 1995; 117:87–103Google Scholar
21 . Yamasaki H, LaBar KS, McCarthy G: Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:11447–11451Google Scholar
22 . Forman SD, Cohen JD, Fitzgerald M, et al: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Google Scholar
23 . Huettel SA, McCarthy G: Evidence for a refractory period in the hemodynamic response to visual stimuli as measured by MRI. Neuroimage 2000; 11:547–553Google Scholar
24 . Kircher TTJ, Rapp A, Grodd W, et al: Mismatch negativity responses in schizophrenia: a combined fMRI and whole-head MEG study. Am J Psychiatry 2004; 161:294–304Google Scholar
25 . Wible CG, Anderson J, Shenton ME, et al: Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res 2001; 108:65–78Google Scholar
26 . Salisbury DF, Shenton ME, Griggs CB, et al: Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry 2002; 59:686–694Google Scholar
27 . Yucel G, Petty C, McCarthy G, et al: Graded visual attention modulates brain responses evoked by task-irrelevant auditory pitch change. J Cogn Neuroscience 2005; 17:1819–1828Google Scholar
28 . Baldeweg T, Klugman A, Gruzelier J, et al: Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia Res 2004; 69:203–217Google Scholar
29 . Oknina LB, Wild-Wall N, Oades RD, et al: Frontal and temporal sources of mismatch negativity in healthy controls, patients at onset of schizophrenia in adolescence and others at 15 years after onset. Schizophrenia Res 2005; 76:25–41Google Scholar
30 . Kapur S: Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23Google Scholar
31 . Lavie N: Distracted and confused? Selective attention under load. Trends Cogn Sci 2005; 9:75–82Google Scholar
32 . Yucel G, Petty C, McCarthy G, et al: Graded visual attention modulates brain responses evoked by task-irrelevant auditory pitch changes. J Cogn Neurosci 2005; 17:1819–1828Google Scholar
33 . Carter CS, Braver TS, Barch DM, et al: Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280:747–749Google Scholar
34 . Carter CS, MacDonald AW III, et al: Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry 2001; 158:1423–1428Google Scholar
35 . Szeszko PR, Ardekani BA, Ashtari M, et al: White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005; 162:602–605Google Scholar
36 . Honea R, Crow TJ, Passingham D, et al: Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005; 162:2233–2245Google Scholar
37 . Friston KJ, Frith CD: Schizophrenia: a disconnection syndrome? Clin Neuroscience 1995; 3:89–97Google Scholar
38 . Huettel SA, Singerman JD, McCarthy G: The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage 2001; 13:161–175Google Scholar
39 . Umbricht D, Bates J, Lieberman J, et al: Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry 2006; 59:762–772Google Scholar
40 . Mathalon DH, Ford JM, Rosenbloom M, et al: P300 reduction and prolongation with illness duration in schizophrenia [erratum in Biol Psychiatry 2000; 47:1091]. Biol Psychiatry 2000; 47:413–427Google Scholar
41 . Olichney JM, Iragui VJ, Kutas M, et al: Relationship between auditory P300 amplitude and age of onset of schizophrenia in older patients. Psychiatry Res 1998; 79:241–254Google Scholar
42 . Johnstone EC, Owens DG, Bydder GM, et al: The spectrum of structural brain changes in schizophrenia: age of onset as a predictor of cognitive and clinical impairments and their cerebral correlates. Psychol Med 1989; 19:91–103Google Scholar
43 . Jeste DV, McAdams LA, Palmer BW, et al: Relationship of neuropsychological and MRI measures to age of onset of schizophrenia. Acta Psychiatrica Scandinavica 1998; 98:156–164Google Scholar
44 . Halgren E, Marinkovic K, Chauvel P: Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 1998; 106:156–164Google Scholar
45 . Mathalon DH, Ford JM, Pfefferbaum A: Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry 2000; 47:434–449Google Scholar
46 . Ford JM, White PM, Csernansky JG, et al: ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry 1994; 36:153–170Google Scholar
47 . Murphy K, Dixon V, LaGrave K, et al: A validation of event-related fMRI comparisons between users of cocaine, nicotine, or cannabis and control subjects. Am J Psychiatry 2006; 163:1245–1251Google Scholar
48 . Zubieta J, Lombardi U, Minoshima S, et al: Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry 2001; 49:906–913Google Scholar