Mercury Exposure: Effects Across the Lifespan
 |
Elemental Mercury
Mercury vapor is highly diffusible and lipid soluble, thus it is easily absorbed. 3 , 7 Initially it distributes principally to red blood cells and then throughout the body. It crosses both the blood-brain and blood-placental barriers. Studies in nonhuman primates of mercury deposition in the cortex following exposure to mercury vapor found a clear laminar pattern in adults, with higher concentrations in pyramidal cells in the deeper layers. 9 Exposure in utero resulted in a lower level of deposition into the brain that was similar in all layers. The dissolved mercury vapor can oxidize to form inorganic mercuric mercury, a process that is inhibited by ethanol. Elimination is slow, with an average half-life of about 8 weeks for the body and possibly years for the brain. 3 , 10 Fecal elimination predominates during the first week, with renal elimination predominating thereafter. Thus, urinary level of mercury (usually expressed as μg Hg/g creatinine or μg Hg/liter urine) is commonly used as an indicator for chronic exposure to mercury vapor and/or inorganic mercury compounds. 11 Unlike methylmercury, these are not readily accumulated into hair. 3 , 11
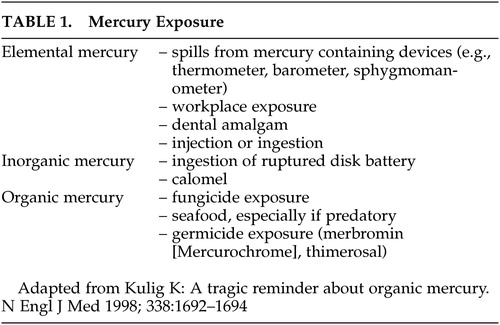
Symptoms of acute intoxication include shortness of breath, chest pain, dyspnea, paroxysmal cough, chills, nausea and vomiting, diffuse joint swelling, and rash. 3 , 12 Chronic exposure commonly results in oral cavity lesions, tremor, decreased coordination, decreased sensation, and psychiatric symptoms including anxiety, excessive timidity, and pathological fear of ridicule ( Figure 1 ). Acrodynia (pink disease), a rare reaction to elemental or inorganic mercury exposure in children, is characterized by sweating, redness and swelling in the hands and feet, progressive weight loss, insomnia, weakness, apathy, hypotonia, hypertension, and tachycardia. 3 , 5 , 13 , 14 The similarity in presentation to pheochromocytoma has been noted. 14 , 15 MRI findings have included hyperintense lesions in multiple regions such as the paracentral area, frontal white matter, and basal ganglia ( Figure 1 ). 12 , 13 A single slice single photon emission computed tomography (SPECT) study in a patient with chronic inorganic mercury poisoning showed increased regional cerebral blood flow (rCBF) in the posterior cingulate. 12
Clear toxicity has been reported for exposures resulting in urinary levels of 50 to 100 μg Hg/liter. The effects of lower levels are still a subject for debate. A recent review of studies of dental professionals, for example, concluded that the evidence for adverse effects in adults associated with urinary levels ≤22 μg Hg/liter was weak. 3 They noted, however, that these studies did not address the possibility of much higher exposures in the past resulting in irreversible effects. The potential toxicity associated with amalgam dental fillings is a particular area of controversy. An autopsy study in adults that measured both organic and inorganic mercury in blood and body tissues estimated an increase of about 1.5 μg Hg/kg of occipital cortex for every 10 amalgam dental fillings. 16 The authors noted that individual differences were considerable, and correlation between number of amalgam fillings and inorganic mercury in the brain accounted for only 30% of the variance. Two recent randomized studies in children compared neurobehavioral measures between groups receiving amalgam and resin composite fillings. 17 , 18 Both studies found significantly increased urinary mercury in the amalgam group at follow-up. In one, measures at the 5-year follow-up examination indicated no significant differences between groups in full-scale IQ (study powered to detect a 3-point reduction) or in tests of memory and visuomotor function. 17 In the other, measures at the 7-year follow-up examination indicated no significant differences in memory, attention, visuomotor function, or nerve conduction velocities. 18 As noted by authors of both studies and in the accompanying editorial, neither study was designed to address effects in potentially more vulnerable populations (e.g., genetic predisposition to mercury toxicity, patients younger than 6 years old). 19
Organic Mercury
Although there are many other organic mercury compounds, most human exposure is to methylmercury, which crosses both the blood-brain and blood-placental barriers and principally damages the CNS. 3 , 20 Ingested methylmercury is distributed first to the blood and then throughout the body, with ∼5% remaining in the blood. The mercury content of red blood cells provides a reliable measure of methylmercury exposure in the absence of significant exposure to inorganic mercury compounds. 11 The ratio of brain to blood concentration is in the range of 5 to 10:1. 21 Elimination is primarily through feces, with a half-life of 6 to 10 weeks. 3 , 21 Methylmercury is also converted to inorganic mercury by intestinal flora. Scalp hair accumulates methylmercury, and analysis of one cm segments is used as a surrogate measure to estimate monthly exposure history for the brain. 3 , 11 , 21 This method is less than ideal, however, as there is no way to differentiate mercury that is within the hair from mercury that is on the surface. 22 Cleaning procedures will not reliably remove surface mercury. 22 , 23 External contamination may occur from a variety of sources, including nearby gold mining and use of mercury-containing personal care products. Analysis of toenail clippings has also been used to estimate exposure to methylmercury. 16
The major route of exposure to methylmercury is through consumption of fish and marine mammals. Inorganic mercury, present in the environment as a result of emissions from both natural (e.g., volcanoes) and human (e.g., coal-burning power stations) sources, is converted to methylmercury within microorganisms in aquatic sediments, allowing it to enter the food chain. Biomagnification occurs at each ascending level of the food chain. Thus, the highest concentrations are found in muscles of longer-living aquatic predators (e.g., pike, shark), principally as a methylmercury cysteine complex. 24 Industrial pollution is another source. It can result in localized areas in which the level of methylmercury in fish is very high, as happened in Minamata Bay in Japan. The other major occurrence of widespread mercury poisoning resulted from the use of seed grain that had been treated with methylmercury fungicide for bread making in rural areas of Iraq. 3 Studies over the past several decades of these two populations have helped to delineate the effects of both adult and prenatal methylmercury exposure. 3 , 25 Methylmercury’s high affinity for thiol groups provides multiple potential targets, and many possible mechanisms for neurotoxicity of methylmercury have been proposed. 26 , 27 Animal studies indicate that methylmercury can induce cell death by both apoptosis and necrosis. It can have multiple deleterious actions at the cellular level, including disruption of microtubules (a key cytoskeletal component), increased generation of reactive oxygen species, and disruption of calcium homeostasis.
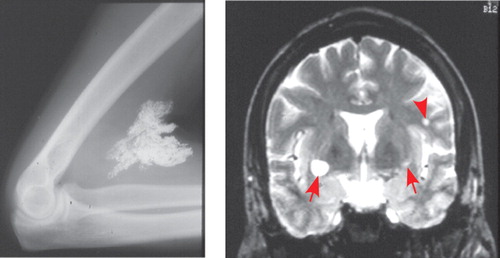
Symptoms of acute exposure to methylmercury in adults (Minamata disease) may include impairments in all primary sensory modalities (blurred vision, with bilateral and symmetric constriction of the visual fields; bilateral deficits in hearing, with speech discrimination more impaired than pure tone; deficits in smell and taste; distal paresthesias with preserved or hyperactive tendon reflexes) as well as dysarthria and cerebellar ataxia. Psychiatric symptoms are common and include loss of volition and apathy, excessive interpersonal sensitivity, perseveration, and loss of inhibition. 3 , 25 , 27 Symptoms may not be immediate, but rather may first occur weeks to months after the exposure. 3 , 27 In one well-documented case, a scientist first displayed clear signs of poisoning 4 months after a single exposure to dimethylmercury. 28 Mercury in this form is easily absorbed through the skin and by inhalation and is highly toxic. Postmortem studies indicate that the most vulnerable sites in the cerebrum are the primary sensory and motor cortices (calcarine region, precentral gyrus, postcentral gyrus, temporal transverse gyrus) ( Figure 2 ). 25 , 27 – 29 Cerebellar granule cells (but not Purkinje cells) are also very vulnerable. Evidence of perivascular edema and demyelination are common. Secondary degeneration of tracts is seen in patients with long survival times. Chronic exposure to methylmercury in adults is most commonly manifested in distal paresthesias. 25 Touch threshold is increased in all areas of the body. Cerebellar ataxia is not usually seen, but sensory ataxia and pseudoathetoid movements may be present. The injury is to the somatosensory cortex, as indicated by preservation of tendon reflexes, normal conduction velocity in the sural nerve, and abnormality of the short-latency somatosensory evoked potential. A SPECT study compared rCBF in elderly patients with mild chronic methylmercury poisoning to age-matched healthy individuals. 30 The groups had similar incidence of white matter abnormalities on MRI. There were no significant differences in cortical rCBF between the groups. Cerebellar atrophy was present in 27% of the patients with methylmercury exposure and none of the participants in the matched comparison group. Regional CBF was significantly decreased in cerebellum even in patients without visible atrophy.
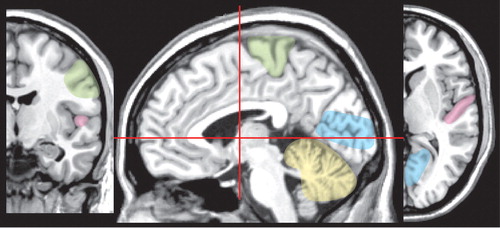
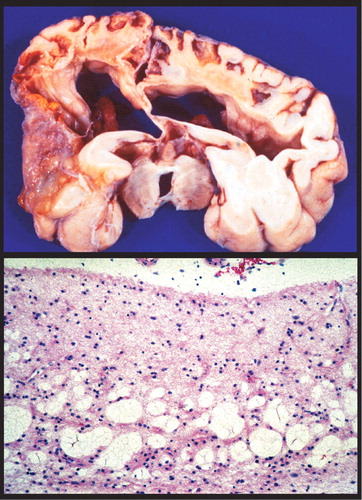
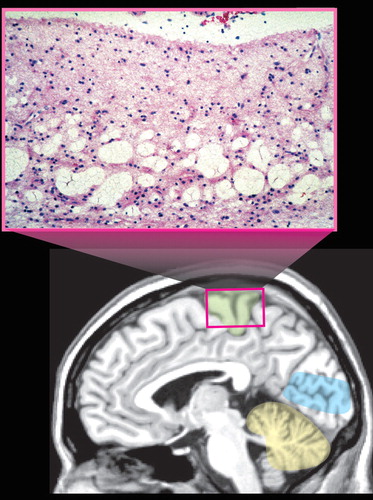
Vulnerability to methylmercury poisoning is age-related, with susceptibility decreasing with increasing age. 2 Exposure to organic mercury compounds during gestation results in severe injury to the infant brain at dose levels below those that are toxic to the mother. 2 , 3 , 20 , 25 , 27 High exposure can result in very severe developmental disorders (e.g., mental retardation, cerebral palsy, blindness, deafness) ( Figure 3 ). 2 , 27 Symptoms of moderate exposure include psychomotor and personality disturbances, intellectual deficits, and epilepsy. Unlike the focal neuropathological changes that are found following adult exposure, prenatal and early childhood exposure results in very widespread changes including status spongiosus of the cerebral cortex ( Figure 3 ). 2 , 3 , 25 , 27 , 29
Lower levels of prenatal exposure are associated with more subtle deficits such as developmental delays. However, differences among the major epidemiological studies complicate identification of lowest-observed adverse effects level. 3 , 20 , 27 One very important difference that makes comparisons across studies difficult is in the type of seafood consumed. In one study, the major source of methylmercury was routine consumption of sea fish (mercury level <0.5 μg/g, 0.3 ppm). In another study, a major source was episodic consumption of pilot whale meat (mercury level up to 3 μg/g, 1.6 ppm) and blubber, resulting in much higher periodic mercury exposures as well as exposure to other toxic substances contained in the blubber. 3 , 31 Competition between positive and negative factors complicates analysis. The possible long-term detrimental effects of occasional exposure to higher levels of mercury and the potentially protective effects of the various nutritional factors contained in fish (e.g., long chain polyunsaturated fats, micronutrients, selenium) may be relevant. 3 , 31 – 35 Other aspects of maternal diet, such as alcohol consumption and fiber intake, may also be important as they can alter absorption and/or elimination of methylmercury. 3
The other organic mercury compound that is presently of great interest is ethylmercury thiosalicylate (thimerosal, Merthiolate), which until recently was commonly used as a preservative in medicines, including vaccines. Use in vaccines was halted in the United States based on lowered exposure limits for methylmercury issued by the Environmental Protection Agency. 36 A recent study in nonhuman primate infants compared brain and blood levels of mercury following exposure to either ethylmercury (intramuscular injection, dosing designed to mimic typical vaccination schedule) or methylmercury (oral, equivalent total dose of mercury). 37 The absorption rates and initial distribution volumes for mercury were similar. Uptake into brain and elimination from the body were quite different. Concentration of mercury in the brain following ethylmercury administration was approximately one-third the level found following methylmercury administration. More than 30% was in the less toxic inorganic form, compared to less than 10% for methylmercury. Clearance of ethylmercury was also two to three times faster than methylmercury for both blood and brain. As noted by the authors, 37 these results indicate that methylmercury is not an appropriate reference for assessing risk related to ethylmercury exposure. These results have been partially confirmed in human infants. 38 The blood half-life of ethylmercury in infants following intramuscular injection of vaccine is much shorter than the blood half-life of oral methylmercury in adults.
The proposed association between thimerosal exposure and autistic spectrum disorder has received extensive attention. 36 As recently reviewed, most epidemiological studies have not found an increased incident of autistic spectrum disorder due to vaccine exposure. 39 A case control study found no significant difference in hair or blood mercury level between children with autistic spectrum disorder and healthy children, although others have disagreed with this analysis. 40 , 41 It has been suggested that some individuals with autistic spectrum disorder may have heightened vulnerability due to impaired elimination of mercury. 42 Mechanisms that have been implicated include lower glutathione levels, altered immune system function, and elevated exposure to oral antibiotics early in life. 43 , 44
Both acute and chronic exposure to toxic substances may contribute to the development of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. 8 , 45 , 46 Injury can occur by direct mechanisms and/or indirectly by altering susceptibility at later ages to disease development. For example, elevated levels of mercury have been found in both blood and brain in patients with Alzheimer’s disease, suggesting that its accumulation may be a factor in the development of this disease. 47 Proposed mechanisms for delayed effects include mercury-induced increased formation of insoluble β-amyloid, mercury-induced increased formation of reactive oxygen species during oxidative stress, excitotoxicity due to inhibition of glutamate reuptake, and increased glial reactivity promoting neuroinflammatory processes. 8 , 47 , 48
In summary, mercury has been known since antiquity to be both a useful and a harmful heavy metal. As one examines patients across the lifespan, significant exposures at younger ages are most devastating. However, chronic lower level exposures at any age can also lead to neuropsychiatric symptomology and reaffirms the necessity to include poison/toxin exposure questions in a clinical interview.
1 . Netscher DT, Friedland JA, Guzewicz RM: Mercury poisoning from intravenous injection: treatment by granuloma resection. Ann Plast Surg 1991; 26:592–596Google Scholar
2 . Davis LE, Kornfeld M, Mooney HS, et al: Methylmercury poisoning: long-term clinical, radiological, and pathological studies of an affected family. Ann Neurol 1994; 35:680–688Google Scholar
3 . Clarkson TW, Magos L: The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 2006; 36:609–662Google Scholar
4 . Alessio L, Campagna M, Lucchini R: From lead to manganese through mercury: mythology, science, and lessons for prevention. Am J Ind Med 2007; 50:779–787Google Scholar
5 . Risher JF, Nickle RA, Amler SN: Elemental mercury poisoning in occupational and residential settings. Int J Hyg Environ Health 2003; 206:371–379Google Scholar
6 . Saper R, Phillips R, Sehgal A, et al: Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the internet. JAMA 2008; 300:915–923Google Scholar
7 . Clarkson TW, Magos L, Myers GJ: The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 2003; 349:1731–1737Google Scholar
8 . Monnet-Tschudi F, Zurich MG, Boschat C, et al: Involvement of environmental mercury and lead in the etiology of neurodegenerative disease. Rev Environ Health 2006; 21:105–107Google Scholar
9 . Warfvinge K, Hua J, Logdberg B: Mercury distribution in cortical areas and fiber systems of the neonatal and maternal adult cerebrum after exposure of pregnant squirrel monkeys to mercury vapor. Environ Res 1994; 67:196–208Google Scholar
10 . Wastensson G, Lamoureux D, Sallsten G, et al: Quantitative assessment of neuromotor function in workers with current low exposure to mercury vapor. Neurotoxicology 2008; 29:596–604Google Scholar
11 . Berglund M, Lind B, Bjornberg KA, et al: Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health 2005; 4:20Google Scholar
12 . O’Carrol RE, Masterton G, Dougall N, et al: The neuropsychiatric sequelae of mercury poisoning: the Mad Hatter’s disease revisited. Br J Psychiatry 1995; 167:95–98Google Scholar
13 . Abbaslou P, Zaman T: A child with elemental mercury poisoning and unusual brain MRI findings. Clin Toxicol (Phila) 2006; 44:85–88Google Scholar
14 . Gattineni J, Weiser S, Becker AM, et al: Mercury intoxication: lack of correlation between symptoms and levels. Clin Pediatr (Phila) 2007; 46:844–846Google Scholar
15 . Torres AD, Rai AN, Hardiek MI: Mercury intoxication and arterial hypertension: report of two patients and review of the literature. Pediatrics 2000; 105:E34Google Scholar
16 . Bjorkman L, Lundekvam BF, Laegreid T, et al: Mercury in human brain, blood, muscle, and toenails in relation to exposure: an autopsy study. Environ Health 2007; 6:30Google Scholar
17 . Bellinger DC, Trachtenberg F, Barregard L, et al: Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA 2006; 295:1775–1783Google Scholar
18 . DeRouen TA, Martin M, Leroux B, et al: Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA 2006; 295:1784–1792Google Scholar
19 . Needlemann HL: Mercury in dental amalgam: a neurotoxic risk? JAMA 2006; 295:1835–1836Google Scholar
20 . Johansson C, Castoldi AF, Onishchenko N, et al: Neurobehavorioural and molecular changes induced by methylmercury exposure during development. Neurotox Res 2007; 11:241–260Google Scholar
21 . Cernichiari E, Myers GJ, Ballatori N, et al: The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology 2007; 28:1015–1022Google Scholar
22 . Nuttall KL: Interpreting hair mercury levels in individual patients. Ann Clin Lab Sci 2006; 36:248–261Google Scholar
23 . Li YF, Chen C, Li B, et al: Scalp hair as a biomarker in environmental and occupational mercury exposed populations: suitable or not? Environ Res 2008; 107:39–44Google Scholar
24 . Harris HH, Pickering IJ, George GN: The chemical form of mercury in fish. Science 2003; 301:1203Google Scholar
25 . Ekino S, Susa M, Nimomiya T, et al: Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 2007; 262:131–144Google Scholar
26 . Boivie J, Leijon G, Johansson I: Central poststroke pain: a study of the mechanisms through analyses of the sensory abnormalities. Pain 1989; 37:173–185Google Scholar
27 . Castoldi AF, Coccini T, Ceccatelli S, et al: Neurotoxicity and molecular effects of methylmercury. Brain Res Bull 2001; 55:197–203Google Scholar
28 . Nierenberg DW, Nordgren RE, Chang MB, et al: Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N Engl J Med 1998; 338:1672–1676Google Scholar
29 . Eto K: Minamata disease. Neuropathology 2000; 20:S14–S19Google Scholar
30 . Itoh K, Korogi Y, Tomiguchi S, et al: Cerebellar blood flow in methylmercury poisoning (Minamata disease). Neuroradiology 2001; 43:279–284Google Scholar
31 . Myers GJ, Davidson PW, Strain JJ: Nutrient and methyl mercury exposure from consuming fish. J Nutr 2007; 137:2805–2808Google Scholar
32 . Davidson PW, Strain JJ, Myers GJ, et al: Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology 2008; 5:767–775Google Scholar
33 . Oken E, Wright RO, Kleinman KP, et al: Maternal fish consumption, hair mercury and infant cognition in a U.S. Cohort. Environ Health Perspect 2005; 113:1376–1380Google Scholar
34 . Rice DC: Overview of modifiers of methylmercury neurotoxicity: chemicals, nutrients and the social environment. Neurotoxicology 2008 Aug 5. [Epub ahead of print]Google Scholar
35 . Strain JJ, Davidson PW, Bonham MP, et al: Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology 2008 Jun 11. [Epub ahead of print]Google Scholar
36 . Baker JP: Mercury, vaccines and autism: one controversy, three histories. Am J Public Health 2008; 98:244–253Google Scholar
37 . Burbacher TM, Shen DD, Liberato N, et al: Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect 2005; 113:1015–1021Google Scholar
38 . Pichichero ME, Gentile A, Giglio N, et al: Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics 2008; 121:e208–e214EGoogle Scholar
39 . Parker S, Todd J, Schwartz B, et al: Thimerosal-containg vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics 2005; 115:793–804Google Scholar
40 . Ip P, Wong V, Ho M, et al: Mercury exposure in children with autistic spectrum disorder: case-control study. J Child Neurol 2004; 19:431–434Google Scholar
41 . DeSoto M, Hitlan RT: Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurol 2007; 22:1308–1311Google Scholar
42 . Mutter J, Naumann J, Schneider R, et al: Mercury and autism: accelerating evidence? Neuro Endocrinol Lett 2005; 26:439–446Google Scholar
43 . Adams JB, Romdalvik J, Ramanujam VM, et al: Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A 2007; 70:1046–1051Google Scholar
44 . Pessah IN, Seegal RF, Lein PJ, et al: Immunologic and neurodevelopmental susceptilities of autism. Neurotoxicology 2008; 29:532–545Google Scholar
45 . Yokel RA: Blood-brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J Alzheimers Dis 2006; 10:223–253Google Scholar
46 . Peterson MS, Weihe P, Choi A, et al: Increased prenatal exposure to methlymercury does not affect the risk of Parkinson’s disease. Neurotoxicology 2008; 29:591–595Google Scholar
47 . Mutter J, Naumann J, Sadaghiani C, et al: Alzheimer Disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuro Endocrinol Lett 2004; 25:331–339Google Scholar
48 . Domingo JL: Aluminum and other metals in Alzheimer’s disease: a review of potential therapy with chelating agents. J Alzheimers Dis 2006; 10:331–341Google Scholar