Gender as a Moderator of Cognitive and Affective Outcome After Traumatic Brain Injury
Kirkness and colleagues 2 assessed the interaction of gender and age in relation to outcome at 3 and 6 months postinjury in a population of 157 TBI patients (124 men, 33 women) using the Extended Glasgow Outcome Scale 3 and the Functional Status Examination. 4 They found that women ages 30 or older had significantly poorer outcome than either males or younger females. There was also a different rate of recovery, with women ages 30 and older showing no improvement between 3 and 6 months postinjury.
The existing research on gender differences has produced equivocal findings. Some studies on gender-related outcomes after serious head injury have shown that women are 1.28 times more likely than men to die of their injuries 5 and 1.57 times more likely than men to be classified in terms of persistent vegetative state or severe neurological disability. 1 Conversely, other studies show that men are more likely to suffer fatality 6 or fail to find any gender association. 7 After moderate to severe injury, research has shown that women had better social outcomes and were more likely than men to return to school or work. 8 Dijkers 9 reported a similar female gender advantage when reviewing literature concerning community integration, while Seibert et al. 10 found that significantly more females (69%) reported a worse overall quality of life than males (21%).
Gender-related outcome research following minor injury has found that women are more vulnerable to early and late postconcussional symptoms, 11 , 12 major depressive disorder, and posttraumatic stress disorder (PTSD). 13 In sports related concussion, Broshek et al. 14 found that female athletes reported more postconcussion symptoms than males and had significantly slower simple and complex reaction times, relative to preseason baseline levels. As a group, women were cognitively impaired approximately 1.7 times more frequently than men following comparable concussion. To complicate things however, the most recent study using the Traumatic Brain Injury Model Systems database (1,331 patients) 15 found that women performed significantly better on an executive function test than men.
The impression that TBI outcome may be worse in women than in men has been supported by multicenter studies 13 showing that women are more likely to report cognitive, affective, and somatic symptoms than men. Meta-analytical research has focused on eight studies employing 20 outcome variables, 16 comprising patients 12 years and older, with follow-up periods ranging from 6 weeks to more than 6 years. Outcome variables included death, days of posttraumatic amnesia, length of hospitalization, return to work, and a number of subjective postconcussion symptoms. The outcome was worse in women than in men for 85% of the measured variables, with an average effect size of −0.15. The only variables in which men demonstrated worse outcomes were return to work, tinnitus, and hearing deficits.
A number of possible explanations have been proposed to account for observed gender difference in TBI outcome, although the exact role of the factors proposed has not yet been fully explored. Gender differences may exist in premorbid IQ, family function, problem-solving skills, education, employment, and socioeconomic status, all of which have been shown to be significant predictors of outcome after TBI. 17 Furthermore, a growing body of evidence suggests that men’s and women’s brains differ in functional organization, 18 with women having more bilateral representation of verbal abilities and performance IQ than men. It is possible therefore, that women’s brains are more likely than men’s brains to be affected by diffuse TBI. 16 Alternatively, gender differences in TBI outcome may result from the different ways in which the two sexes experience and report illness and symptoms in general. Epidemiological findings point to a female preponderance in prevalence, incidence, and morbidity risk for a variety of psychosomatic and mental health conditions. 19 Finally, results of animal studies indicate that an interaction of TBI sequelae with gonadal hormones may explain differences in TBI outcome between men and women. One study found that estrogen treatment in rats, before inflicting an experimental fluid-percussion brain injury, provided protective effects in males and exacerbated the injury in females. 20 Similarly, in hyponatremic conditions, depressed oxygen use and cerebral blood flow were observed in female rodents. 21 More recent research, however, has shown that estrogen provided a neuroprotective effect, resulting in a better outcome for female mice compared with males following experimental TBI, inflicted using the murine impact-acceleration head injury method. 22
In spite of these findings and numerous calls for gender differences in TBI to be further explored and articulated, 23 few studies have been specifically designed to study the effect of gender on TBI outcome. The purpose of our investigation was to compare cognitive and affective functions using a prospective matched-groups design in men versus women after TBI. On the basis of previous research, the following hypotheses were formulated: females would be overall more cognitively impaired than males (however, there was no basis for predicting differential impairment in specific functions), and females would report worse affective functioning compared to their male counterparts.
METHODS
Ethical approval for this study was obtained from the Research Ethics Committee of the Department of Psychology, Swansea University. All participants gave their informed consent prior to their inclusion in the study, in compliance with regulations of the institution and the guidelines of the Helsinki Declaration.
Participants
We evaluated 520 consecutive head injured patients (162 women, 358 men) referred between January 2001 and December 2004 to Swansea University Head Injury Clinic for routine neuropsychological assessment, for possible inclusion to the study. Exclusion criteria for this study were: previous history of head injury, neurological disorder, or psychiatric disorder; previous history of a serious medical condition (e.g., cardiovascular disease, chronic kidney disease); alcohol or drug abuse; and speech, motor, or perceptual deficit likely to interfere with neuropsychological assessment. We excluded 143 cases from the cohort based on those criteria. For both men and women the most common mechanism of traumatic brain injury was motor vehicle accident (70%), followed by falls (15%), assault (8%), and pedestrian injury (7%).
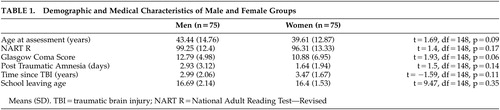
One hundred fifty female and male patients were individually matched on the basis of age (range=20–60 years); severity of injury as indexed by both Post Traumatic Amnesia (PTA) 23 (range=0–110 days) and Glasgow Coma Score (GCS) (range=3–15); 24 premorbid IQ, estimated by the National Adult Reading Test—Revised (NART-R) (range=70–126); 25 and time since injury (range=185–1,460 days). Further, to be included in the study patients had to have an abnormal CT scan. An ideal match was defined as being within an age difference of 5 years (age range=18–70); within five IQ points; in the same severity category, measured by both Post Traumatic Amnesia and Glasgow Coma Score (i.e., PTA classification <1 day minor, 1–7 days severe, >8 days very severe; GCS classification >13 mild, 9–12 moderate, <9 severe); and either less than 2 years or more than 2 years since injury. Where an ideal match could not be found, one of these criteria was extended (within 10 years of age, n=1; within 10 IQ points, n=3).
Participants’ Glasgow Coma scores on admission to the hospital were retrieved from hospital records, and Post Traumatic Amnesia duration was ascertained retrospectively during the course of clinical interview, according to the guidelines proposed by McMillan and colleagues 24 (i.e., last memory prior to incident, first memory following the incident, and assessment for return of continuous day-to-day memory). All participants had abnormal CT scans, indicating frontal hemorrhagic or contusional injuries: 53 left frontal injuries (26 female), 61 right frontal injuries (30 female), and 36 bilateral injuries (17 female). Table 1 summarizes the participants’ demographic and medical characteristics. Matching resulted in homogeneous groups of patients; there were no significant differences among the two groups in demographic and medical characteristics.
 |
Design
This is a prospective matched cohort study. To reduce the “noise” associated with a heterogenous condition such as brain injury and to eliminate potential confounders, one-to-one matching was used to compare male versus female groups. The matching reduces the numbers of covariates needed for statistical analysis, thus simplifying and strengthening the modeling process. 26
Measures
Neuropsychological Assessment
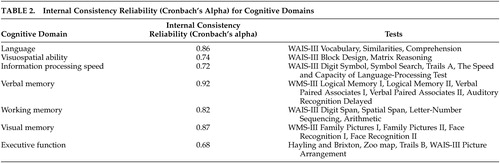
A clinical neuropsychologist (RLW) administered to the patients an extensive battery of tests grouped a priori into cognitive domains on the basis of theory 27 and research, as applied to brain injury. 28 The neuropsychological battery consisted of: the WAIS-III, 29 the Wechsler Memory Scale, 3rd edition (WMS III), 30 the National Adult Reading Test—Revised, 25 the Hayling and Brixton Tests, 31 the Trail Making Test, 32 the Speed and Capacity of Language-Processing Test, 33 and one test from the Behavioral Assessment of the Dysexecutive Syndrome battery. 34 The neuropsychological tests are presented in Table 2 , organized by cognitive domains and the associated internal consistency reliabilities (Cronbach alpha for standardized variables). In general, the internal consistency reliabilities of the domains were excellent. The relatively lower reliability (alpha) for executive function is more likely due to well-documented difficulties in the assessment of this function. 35 , 36
 |
Affective Functioning
To assess depression and anxiety the patients were administered the Beck Depression Inventory, 2nd Edition (BDI-II), 37 and the Beck Anxiety Inventory, 38 respectively.
Statistical Analysis
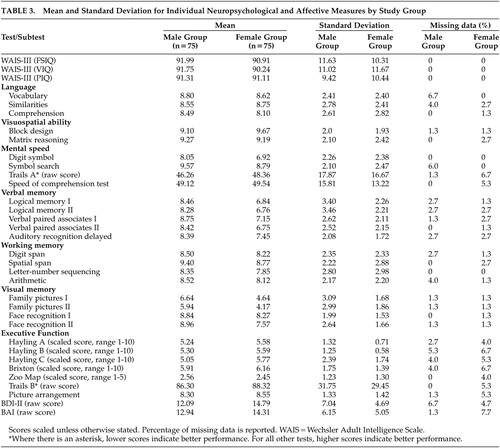
An a priori power analysis indicated greater than 85% power to detect differences of magnitude 0.25 between groups for a sample size of 146 [effect size (medium)=0.25, F=3.91, df=1, 144, λ=9.12]. All analyses were two-tailed, with α level set at 0.05. Type I error was controlled using the Bonferroni’s correction for each family of tests. For both groups, the percentage of scattered missing data for the continuous independent variables ranged approximately from 1.3% to 7.7% ( Table 3 ). Data were found to be missing completely at random as determined by Little’s test 39 (female group: χ 2 =1,183.761, df=1,215, p>0.05; male group: χ 2 =1,622.227, df=1,524, p>0.05). Missing values for the continuous independent variables were imputed using the expectation maximization method. 40 This procedure uses the expectation-maximization algorithm 41 to estimate means, variances, and covariances among the manifest variables. The expectation-maximization algorithm is a preferred method of imputation largely because it uses an iterative process to improve the prediction of missing values as more missing data are imputed. 41
 |
To examine the effects of gender on cognition while controlling for multiple domains and intercorrelations among these domains, a multivariate analysis of variance (MANOVA) was conducted on all cognitive scores simultaneously. When necessary (i.e., with Trails A and B tests) the inverse value was entered into the MANOVA to ensure that higher values consistently indicated better performance. To produce commensurability of the dependent variables, age-corrected z scores based on published normative data were used. In this case the z score for each participant represents the extent to which that participant deviates from the norms for his or her age. The mean of the participant’s age-appropriate reference group based on the standardization sample was subtracted from the participant’s test score and the result was divided by the standard deviation of the participant’s age-appropriate reference group, giving a z score for each test score. Normative data were culled from test manuals. 31 – 34 WAIS III and Wechsler Memory Scale (WMS III) z scores were calculated from the formulas and respectively. Domain scores were then computed by averaging the z scores for all tests available for each participant represented in that composite and the MANOVA was performed on these scores. The effect of age at the time of injury on cognitive function decline was examined with Pearson’s product-moment correlations. Cognitive decline was calculated as the difference score of the National Adult Reading Test—Revised minus the WAIS III full scale IQ score. We conducted a MANOVA on the combined Beck Anxiety Inventory and BDI-II variables to examine the effects of gender on affective functioning. All analyses were conducted using SPSS version 13 (SPSS Inc., Chicago, 2004).

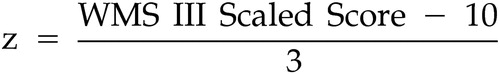
RESULTS
Table 3 provides mean scores and standard deviations for all the neuropsychological and affective measures.
Neuropsychological Functioning
The MANOVA indicated significant differences between groups on the combined dependent variables (F=5.02, df=7, 47, p<0.05, Wilk’s λ=0.57, η p2 =0.43). When the results for the cognitive domains were considered separately, the differences that reached statistical significance, using a Bonferronni adjusted level of 0.008, were verbal memory (F=11.92, df=1, 53, p<0.007, η p2 =0.18) and visual memory (F=10.46, df=1, 53, p<0.007, η p2 =0.16), with women performing at a lower level (z≥1 SD) compared with men (the same pattern of results remained when a MANCOVA was performed with levels of depression as a covariate). Figure 1 depicts the profile of performance of each group across each cognitive domain. Interestingly, the neuropsychological profile of these young women, with memory performance more than 1 SD below age-specific norms, preserved general cognitive functioning, intact ability to perform activities of daily living, and the presence of complaints about memory (the latter two pieces of information were gathered during the course of a short clinical interview before the testing) is reminiscent of mild cognitive impairment. 42
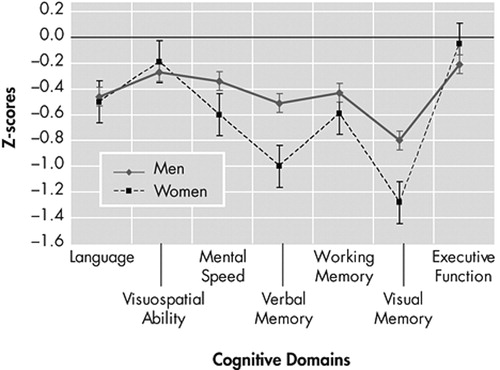
Standard error bars are displayed. The names of the tests represented in the cognitive domains are listed in Table 2.
The Effects of Age on Cognitive Decline by Gender
The correlation between age and cognitive decline was significant for women (r= 0.25, p<0.05), but not for men (r=0.08, p>0.05).
Affective Functioning
Women had marginally elevated anxiety and depression scores compared to men. However, neither of the groups scored in the clinically significant region. The MANOVA indicated significant differences between groups on the combined dependent variables (F=4.77, df=2, 147, p<0.05, Wilk’s λ=0.94, η p2 =0.06). When the results for anxiety and depression were considered separately, using a Bonferronni adjusted level of 0.025, only the differences in depression levels reached statistical significance (F=7.13, df=1, 148, p<0.025, η p2 =0.05).
DISCUSSION
This study aimed to establish whether there are significant differences in the neuropsychological and affective outcomes between men and women who suffer a TBI of comparable severity. The results suggest that men and women experience different cognitive and affective impairments after TBI that cannot be attributed to demographic, psychiatric, and injury severity variables. In support of the first hypothesis, and in line with studies that have reported gender differences in TBI outcomes, 2 , 14 , 16 women were significantly more impaired in verbal and visual memory compared with men. The reasons for this are not clear and we can only speculate about the mechanisms involved. One possible explanation involves the role of the hippocampus. Recent investigations have revealed that in cases of TBI, index scores from the Wechsler Memory Scale, Revised, have correlated with hippocampal volume, 43 and that reductions in hippocampal volumes are significantly associated with impaired memory functions. 44 , 45 Although neurobiological studies have shown a number of mechanisms through which estrogen might protect the hippocampus from dementia and cognitive aging, experimental studies point to sexual dimorphism in hippocampal morphology and neuronal anatomy. 46 , 47 Using an animal model, Shors et al. 47 found that males and females in unstressed conditions have different levels of dendritic spine density in the hippocampus and also that their neural anatomy can respond in opposite directions to the same stressful event. Twenty-four hours after exposure to a relatively short (30 minutes) yet fearful event of brief intermitted tail shocks, the density of the spines on apical dendrites in the male hippocampus increased by as much as 30%, whereas that of females in proestrus was reduced.
In this study the degree of cognitive decline was positively correlated with age at the time of injury in women but not in men. The role of estrogen and progesterone is a possible explanation for this result. Animal models of stroke and TBI have provided evidence to suggest that these hormones may confer a neuroprotective and neuroregenerative effect. 48 The significant correlation supports the hypothesis that gonadal steroids offer protection against neuronal damage in women. Although specific information about menopausal status was not collected in this study, 34.7% of the females in the sample were estimated to be in the perimenopausal, menopausal, and postmenopausal period (45–60 years old). 49 It is possible that, with increasing age and lower levels of circulating female hormones, the effects of TBI are more severe. Future studies are needed to consider the potential interaction of gender, age, and menopausal status (taking into account information about contraceptive and hormone replacement therapy) following TBI.
Another possible explanation for greater impairment of memory in female participants would be that increased levels of anxiety and depression adversely affected cognitive function. It is documented that depression can disrupt encoding and retrieval of information, and impair attentional skills and executive function. 50 , 51 However, although marginally worse than men, ratings of anxiety and depression did not reach clinically significant levels. Therefore, although the results show consistency with past investigations 3 , 13 they only offer partial support to the second hypothesis. Moreover, females only performed significantly worse than their male counterparts on measures of verbal and visual memory, with no significant group differences on measures of attention and executive function (which could be expected if a disorder of affect was implicated in the impaired performance).
Although no significant differences were found between men and women with respect to pathology on initial CT scan, future studies need to focus on whether there are systematic biological sex differences in the type of injury sustained following TBI (e.g., shear injury, intracerebral hematoma), in addition to differences in severity. Similarly, differences in how males and females report symptoms must be further explored because of their potential influence on subjective measures. Another major area for future investigation is the relationship between cognitive and emotional sequels of TBI and functional outcome
Unlike the present study, some studies have failed to find any gender differences in outcome after TBI. 52 , 53 One possible methodological difference distinguishing this from other studies is that none of the previous studies have matched male and female patients, which is important because the variability of within-gender cognitive impairment found in patients is greater than differences between the sexes. Therefore, small gender effect sizes makes it necessary to control for as much extraneous variation as possible, otherwise the significance of gender effects may not be detected, even with large study groups. Also, the 2:1 male-female gender ratio seen in cases of head trauma makes it difficult for the effect of gender to be detected in regression techniques. Although this is a methodological strength of the present study, it also needs to be acknowledged that matched cohorts may not be representative of the brain injury population as a whole because matching can introduce selection bias. When groups are matched on certain confounder measures, this distorts them to be similar with regard to matching variables and perhaps other variables as well. 54 Consequently, the selected cases may not represent the entire population from which they were derived.
Despite these shortcomings, a primary contribution of this study is the evidence it provides for differential cognitive impairment in females who sustain a TBI. The clinical implications of this study are important. Barnes and colleagues 55 and Bounds and colleagues 56 reported severity of injury, gender and primary insurance provider as significant predictors of rehabilitative service utilization. Although the designs of these studies do not allow insight into what factors may affect the likelihood of receiving rehabilitation services following TBI, it seems that men receive more therapy than women. The present investigation points to a need for more provision of memory related rehabilitation in women.
Additional studies are needed to replicate and extend the present findings. A more complete picture of the natural history of gender-related outcomes could be provided by following individuals over long periods of time, examining cognitive, psychosocial and functional outcomes 57 , 58 but with an emphasis on how gender might be a factor determining outcome. Neuroimaging studies are needed to confirm the tentative hypothesis proposed by this study, that women suffer increased hippocampal damage compared to men for injuries of similar severity, and consequently exhibit more memory and affective impairment. A better understanding of factors such as gender that moderate outcomes of TBI would be useful in identifying high risk individuals and in enhancing awareness of ways to facilitate their recovery.
1 . Kraus JF, McArthur DL: Incidence and prevalence of, and costs associated with, traumatic brain injury, in Rehabilitation of the Adult and Child with Traumatic Brain Injury, 3rd ed. Edited by Rosenthal M, Griffith ER, Kreutzer JS. Philadelphia, FA Davis, 1998, pp 3–18Google Scholar
2 . Kirkness CJ, Burr RL, Mitchell PH, et al: Is there a sex difference in the course following traumatic brain injury? Biol Res Nurs 2004; 5:299–310Google Scholar
3 . Levin HS, Boake C, Song J, et al: Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J Neurotrauma 2001; 18:575–584Google Scholar
4 . Dikmen S, Machamer J, Miller B, et al: Functional status examination: a new instrument for assessing outcome in traumatic brain injury. J Neurotrauma 2001; 18:127–140Google Scholar
5 . Klauber MR, Marshall LF, Luerssen TG, et al: Determinants of head injury mortality: importance of the low risk patient. Neurosurgery 1989; 24:31–36Google Scholar
6 . Gujral IB, Stallones L, Gabella BA, et al: Sex differences in mortality after traumatic brain injury, Colorado 1994–1998. Brain Inj 2006; 20:283–291Google Scholar
7 . Coimbra R, Hoyt DB, Potenza BM, et al: Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma 2003; 54:689–700Google Scholar
8 . Groswasser Z, Cohen M, Keren O: Female patients recover better than males. Brain Inj 1998; 12:805–808Google Scholar
9 . Dijkers M: Measuring the long-term outcomes of traumatic brain injury: a review of the community integration questionnaire. J Head Trauma Rehabil 1997; 12:74–91Google Scholar
10 . Seibert PS, Reedy DP, Hash J, et al: Brain injury: quality of life’s greatest challenge. Brain Inj 2002; 16:837–848Google Scholar
11 . Bazarian JJ, Wong T, Harris M, et al: Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj 1999; 13:173–189Google Scholar
12 . Rutherford WH, Merrett JD, McDonald JR: Symptoms at one year following concussion from minor head injuries. Brain Inj 1978; 10:225–230Google Scholar
13 . Levin HS, Mattis S, Ruff RM, et al: Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurgery 1987; 66:234–243Google Scholar
14 . Broshek DK, Kaushik T, Freeman JR, et al: Sex differences in outcome following sports-related concussion. J Neurosurg 2005; 102:856–863Google Scholar
15 . Niemeier JP, Marwitz JH, Lesher K, et al: Gender differences in executive functions following traumatic brain injury. Neuropsychol Rehabil 2007; 17:293–313Google Scholar
16 . Farace E, Alves WM: Do women fare worse: a meta-analysis of gender differences in traumatic brain injury outcome. J Neurosurg 2000; 93:539–545Google Scholar
17 . Rao V, Lyketsos C: Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics 2000; 41:95–103Google Scholar
18 . Farace E, Turkheimer E: Gender differences in brain morphometry and function, in Neuroimaging: Handbook of Brain Function. Edited by Bigler ED. New York, Plenum, 1996, pp 127–151Google Scholar
19 . Piccinelli M, Wilkinson G: Gender differences in depression, critical review. Br J Psychiatry 2000; 177:486–492Google Scholar
20 . Emerson CS, Headrick JP, Vink R: Estrogen improves biochemical and neurologic outcome following traumatic brain injury in male rats, but not in females. Brain Res 1993; 608:95–100Google Scholar
21 . Kozniewska E, Roberts TPL, Vexler ZS, et al: Hormonal dependence of the effects of metabolic encephalopathy on cerebral perfusion and oxygen utilization in the rat. Circ Res 1995; 76:551–558Google Scholar
22 . Kupina NC, Detloff MR, Bobrowski WF, et al: Cytoskeletal protein degradation and neurodegeneration evolves differently in males and females following experimental head injury. Exp Neurol 2003; 180:55–72Google Scholar
23 . Kraus JF, Peek-Asa C, McArthur D: The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurg Focus 2000; 8:E5Google Scholar
23 . Teasdale G, Jennett B: Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–83Google Scholar
24 . McMillan TM, Jongen ELM, Greenwood RJ: Assessment of post-traumatic amnesia after severe closed head injury: retrospective or prospective? J Neurol Neurosurg Psychiatry 1996; 60:422–427Google Scholar
25 . Nelson H, Willison J: National Adult Reading Revised Test Manual. Nfer-Nelson, Windsor, 1991Google Scholar
26 . Cummings P, McKnight B, Greenland S: Matched cohort methods for injury research. Epidemiologic Reviews 2003; 25:43–50Google Scholar
27 . Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
28 . Wood RL, Liossi C: Neuropsychological and neurobehavioural correlates of aggression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 2006; 18:333–341Google Scholar
29 . Wechsler D: Wechsler Adult Intelligence Scale, 3rd edition. San Antonio, Tex, The Psychological Corporation, 1997Google Scholar
30 . Wechsler D: Wechsler Adult Memory Scale, 3rd ed. The Psychological Corporation, San Antonio, Tex, 1997Google Scholar
31 . Burgess PW, Shallice T: The Hayling and Brixton Tests. Bury St. Edmunds, UK, Thames Valley Test Company, 1997Google Scholar
32 . Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, Ariz, Neuropsychology Press, 1985Google Scholar
33 . Baddeley A, Emslie H, Smith IN: The Speed and Capacity of Language Processing Test. Bury St. Edmunds, Thames Valley Test Company, 1992Google Scholar
34 . Wilson BA, Alderman N, Burgess PW, et al: Behavioural Assessment of the Dysexecutive Syndrome. Bury St. Edmunds, Thames Valley Test Company, 1996Google Scholar
35 . Wood RL, Liossi C: The ecological validity of executive tests in a severely brain injured sample. Arch Clin Neuropsychol 2006; 21:429–437Google Scholar
36 . Wood RL, Liossi C: The relationship between general intellectual ability and performance on ecologically valid executive tests in a severe brain injury sample. J Int Neuropsychol Soc 2007; 13:90–108Google Scholar
37 . Beck AT, Steer RA, Brown GK: Manual for Beck Depression Inventory II (BDI-II). San Antonio, Tex, Psychology Corporation, 1996Google Scholar
38 . Beck AT, Epstein N, Brown G, et al: An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Google Scholar
39 . Little R: A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988; 83:1198–1202Google Scholar
40 . Dempster AP, Laird NM, Rubin DB: Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc B 1977; 39:1–38Google Scholar
41 . Little RJ, Schenker N: Missing data, in Handbook of Statistical Modelling for the Social and Behavioural Sciences. Edited by Arminger GH, Clogg CC, Sobel ME. New York, Plenum, 1995, pp 39–75Google Scholar
42 . Ritchie K, Touchon J: Mild cognitive impairment: conceptual basis and current nosological status. Lancet 2000; 355:225–228Google Scholar
43 . Tate DF, Bigler ED: Fornix and hippocampal atrophy in traumatic brain injury. Learn Mem 2000; 7:442–446Google Scholar
44 . Bigler ED, Anderson CV, Blatter DD, et al: Temporal lobe morphology in normal aging and traumatic brain injury. Am J Neuroradiol 2002; 23:255–266Google Scholar
45 . Himanen L, Portin R, Isoniemi H, et al: Cognitive functions in relation to MRI findings 30 years after traumatic brain injury. Brain Injury 2005; 19:93–100.Google Scholar
46 . Hortnagl H, Hansen L, Kindel G, et al: Sex differences and estrous cycle-variations in the AF64A-induced cholinergic deficit in the rat hippocampus Brain Res Bull 1993; 31:129–134Google Scholar
47 . Shors TJ, Chua C, Falduto J: Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 2001; 21:6292–6297Google Scholar
48 . Wise PM, Dubal D, Wilson ME, et al: neuroprotective effects of estrogen—new insights into mechanisms of action. Endocrinology 2001; 142:969–973Google Scholar
49 . Whwlan EA, Sandler DP, McConnaughey DR, et al: Menstrual and reproductive characteristics and age at natural menopause. Am J Epidemiol 1990; 131:625–632Google Scholar
50 . Hart RP, Kwentus JA, Hamer RM, et al: Selective reminding procedure in depression and dementia. Psychol and Aging 1987; 2:111–115Google Scholar
51 . Rapoport MJ, McCullagh S, Shammi P, et al: Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci 2005; 17:61–65Google Scholar
52 . Wood RL, Rutterford NA: Demographic and cognitive predictors of long term psychosocial outcome following traumatic brain injury. J Int Neuropsychol Soc 2006; 12: 350–358Google Scholar
53 . Rutterford NA, Wood RL: Evaluating a Theory of Stress and Adjustment When Predicting Long Term Psychosocial Outcome after Brain Injury. J Int Neuropsychol Soc 2006; 12:359–367. Google Scholar
54 . Rothman KJ: Modern Epidemiology . Boston, Little, Brown, 1986 Google Scholar
55 . Barnes E, Franck EM, Montgomery A, et al: Factors predicting rehabilitative service provision in adults with traumatic brain injury. J Med Speech-Language Pathology 2005; 13:69–84Google Scholar
56 . Bounds TA, Schopp L, Johnstone B, et al: Gender differences in a sample of vocational rehabilitation clients with TBI. NeuroRehabilitation 2003; 18:189–196Google Scholar
57 . Wood RL, Rutterford NA: Long-term effect of head trauma on intellectual abilities: a 16-year outcome study. J Neurol Neurosurg Psychiatry 2006; 77:1180–1184Google Scholar
58 . Wood RL, Rutterford NA: Psychosocial adjustment 17 years after severe brain injury. J Neurol Neurosurg Psychiatry 2006; 77:71–73Google Scholar



