The Role of Mild Depression in Sleep Disturbance and Quality of Life in Parkinson’s Disease
Efforts directed at addressing the neuropsychiatric features early in Parkinson’s disease are supported by a large body of research in neuropsychiatry that is focused on early intervention for potentially modifiable causes of symptoms associated with dementia. 5 In Parkinson’s disease, the need for such approaches is exemplified by studies showing that depression and apathy, anxiety, sleep disturbances, and hallucinations 6 – 8 are predictive of cognitive decline and are risk factors for Parkinson’s disease dementia. The relationship between these neuropsychiatric features and cognitive impairment is evident even in nondementia samples, suggesting that early screening and management of these symptoms are warranted.
Depression and sleep disturbance in Parkinson’s disease are two symptoms that have been shown to be both prodromal features 9 and predictors of cognitive decline and reduced quality of life longitudinally. 10 , 11 This suggests that they may be suitable targets for early intervention. Recent systematic reviews have estimated the prevalence of major depression in Parkinson’s disease to be 17% to 25%, while clinically significant depressive symptoms may occur in up to 50% of patients. 12 Sleep disturbance is also extremely common, affecting around two-thirds of patients, 13 and may be related to REM sleep behavior disorder, restless legs syndrome, medication effects, pain, and discomfort. A recent review indicated that insomnia and excessive daytime sleepiness occur in around 60% and 30% of patients, respectively, and are influenced by depressed mood. 14
Delineation of the interrelationships between depression and sleep disturbance in Parkinson’s disease is supported by literature in other aging samples that shows that these features are associated with cognitive decline and/or dementia 15 and increased mortality. 16 , 17 Recent large studies of older community-dwelling adults suggest that sleep disturbance is also a risk factor for depression onset and relapse. 18 Importantly, however, both can be managed using pharmacological and nonpharmacological therapies. Additionally, there is considerable data to suggest that untreated depression in older adults is associated with cognitive decline 5 and that newer generation antidepressants are neuroprotective, perhaps acting through the promotion of key neurotrophic factors integral for neurogenesis. Unfortunately, despite the significant impact of depression on quality of life and disability in Parkinson’s disease, data indicate that these symptoms are often under-recognized and undertreated. 19
Prior research has demonstrated that depression in Parkinson’s disease predicts impaired social, role, and physical functioning, independent of the impact of illness severity. 20 Furthermore, population-based studies have shown that depression accounts for 54% of the variance in quality of life scores, 21 and several reports have indicated that the impact of depression on quality of life exceeds that of the motor aspects of the disease. 3 , 21 Similarly, a longitudinal study of 82 patients in later disease stages 10 demonstrated that baseline levels of depression and insomnia were significant predictors of health-related quality of life over an 8-year period. However, sleep disturbance was not assessed comprehensively, nor did this study employ scales developed specifically for Parkinson’s disease. Due to the etiological complexity and range of sleep disturbances in Parkinson’s disease, comprehensive, disease-specific, and well-validated tools are required to capture both daytime sleepiness and nocturnal disturbance. A recent analysis of the Parkinson’s Disease Sleep Scale and the Scales for Outcomes in Parkinson’s Disease—Sleep Scale (SCOPA-S) has validated both tools as being capable of assessing these features of disease. This study showed depression and anxiety symptoms were major predictors of nocturnal sleep disturbance, while disease severity was not a significant predictor. Sleep disturbance was only weakly correlated with health-related quality of life. 22
Overall, these studies suggest that sleep disturbance and depression may overlap and arise from common neurobiological networks, yet both may differentially contribute to quality of life. However, prior research lacked the inclusion of both sleep and quality of life measures developed specifically for use in Parkinson’s disease cohorts. Additionally, previous studies have not commonly considered how sleep and depression are interrelated or, conversely, how they may contribute independently to quality of life. Characterization of these features early in the disease course is warranted in order to inform the timing and delivery of targeted interventions. Therefore, in this study we aimed to evaluate the relative contributions of depression and disease severity to sleep disturbance and to evaluate the relative contributions of sleep, disease severity, and depression to quality of life.
METHODS
Participants
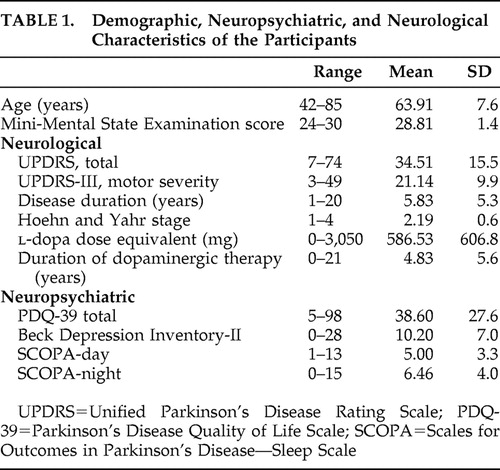
Thirty-five patients (mean age=63.9 years [SD=7.6]; 19 men) with idiopathic Parkinson’s disease were recruited from the Brain & Mind Research Institute (BMRI) Parkinson’s Disease Research Clinic at the University of Sydney in Australia. All patients satisfied United Kingdom Parkinson’s Disease Society (UKPDS) Brain Bank criteria and were assessed on their regular medication. All patients underwent careful historical review along with physical examination. This study was approved by the ethics committee of the University of Sydney, and all patients gave written informed consent.
Measures
Neurological
All patients received a neurological examination, were rated as between Hoehn and Yahr stages I to IV (mean stage=2.2, SD=0.6) and were assessed on sections I–V of the Unified Parkinson’s Disease Rating Scale (UPDRS). 23 Section III was used for analysis of motor severity (UPDRS-III). Mean disease duration was 5.8 years (range=1–20 [SD=5.3]). Six patients were untreated. The other 29 were receiving l -dopa, of whom 11 were also receiving a dopamine agonist, seven were receiving entacapone, one was receiving benztropine, three were on amantadine, and two had undergone previous bilateral subthalamic nucleus deep brain stimulation (STN-DBS). Five patients were taking a selective serotonin reuptake inhibitor for mood, and another was on a tricyclic to aid sleep. Finally, three patients were taking a nocturnal benzodiazepine. l -dopa dose equivalents were calculated according to published criteria, 24 and the number of years of l -dopa treatment was recorded.
Depression, Sleep Disturbance, and Quality of Life
Depressive symptoms were self-rated using the Beck Depression Inventory—II (BDI-II). 25 On this questionnaire, 21 items are rated on a 4-point scale (maximum=63) with score ranges of 0–13, 14–19, 20–28, and 29–63 denoting minimal, mild, moderate, and severe depression, respectively. The BDI-II shows good psychometric properties for older adults and is a commonly used tool for use in Parkinson’s disease with adequate test-retest reliability and validity compared to a DSM-IV diagnosis of depression. 26 To assess levels of sleep disturbance, patients completed the Scales for Outcomes in Parkinson’s Disease—Sleep Scale (SCOPA-S). 27 This scale addresses sleep problems over the past month using four response options and consists of a nighttime (SCOPA-night; maximum=15) and daytime (SCOPA-day; maximum=18) scale. Recent data suggest that SCOPA-S has adequate psychometric properties for assessing sleep disturbance in Parkinson’s disease. Quality of life was measured using the Parkinson’s Disease Quality of Life Scale (PDQ-39), which was developed and validated specifically for people with Parkinson’s disease. 28 Recent analyses suggest that it has good reliability and acceptability for the overall summed items. 29 Scores range from 0 to 156 with higher scores indicating a poorer quality of life.
Statistical Analysis
Data were analyzed using SPSS version 16 for Macintosh and employed Pearson correlation coefficients for univariate analyses. Multivariate analyses used multiple linear regression with forced entry of all relevant variables to determine the semipartial (part) correlation associated with each variable after controlling for other predictors. All analyses used an alpha of 0.05 and were two-tailed.
RESULTS
Descriptive statistics for the sample are displayed in Table 1 . Disease severity was mild to moderate. Levels of depressive symptomatology ranged from normal to moderate, with mean scores in the minimal depression range.
 |
Daytime Sleepiness
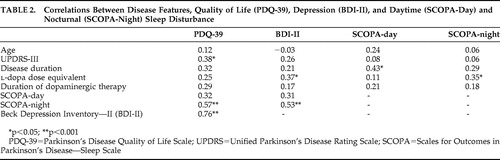
Table 2 shows that the relationship between daytime sleepiness and age was not significant. There was no significant association between SCOPA-day scores and motor disease severity, l -dopa dose equivalent, duration of dopaminergic therapy, or BDI-II scores. However, greater daytime sleepiness was significantly related to longer disease duration. There was a low to moderate correlation between SCOPA-day and SCOPA-night scores (r=0.34, p<0.05).
 |
Nocturnal Sleep Disturbance
Table 2 shows that nocturnal sleep disturbance (SCOPA-night) was not significantly related to age, UPDRS-III, disease duration or duration of dopaminergic therapy. However, there was a significant relationship between greater nocturnal sleep disturbance and higher l -dopa dose equivalents, as well as more severe depressive symptoms as recorded by the BDI-II.
Quality of Life
In univariate analyses, PDQ-39 scores were unrelated to age, disease duration, l -dopa dose equivalent, or duration of dopaminergic therapy ( Table 2 ). Higher PDQ-39 scores were significantly related to greater nocturnal sleep disturbance, as well as greater motor disease and depression severity.
In a multivariate model with PDQ-39 as the dependent variable and with forced entry of SCOPA-night, UPDRS-III, and BDI-II, all three variables remained significant and overall predicted 67.7% of the variance in PDQ-39 scores (F=21.66, df=3, 31, p<0.001). BDI-II scores were the largest independent predictor, uniquely explaining 20.8% of the variance (t=4.5, p<0.001). UPDRS-III scores uniquely predicted 5.7% of the variance (t=2.3, p<0.05), and SCOPA-night scores uniquely predicted 4.6% of the variance in PDQ-39 scores (t=2.1, p<0.05), leaving 36.6% as shared predictor variance. Since the PDQ-39 contains six items pertaining to mood and emotional functioning, this analysis was repeated with their exclusion. The resultant analysis did not change appreciably (BDI-II: t=4.1, p<0.001; UPDRS-III: t=2.1, p<0.05; SCOPA-night: t=2.1, p<0.05), thereby affirming that the predictive capacity of the BDI-II was not merely due to overlap of similar items.
DISCUSSION
This study uses disease-specific measures to examine the interrelationships between sleep disturbance, depressive symptoms, disease severity, and quality of life in Parkinson’s disease. The findings demonstrate that daytime sleepiness was not associated with depression or severity of motor symptoms but was predicted by greater disease duration and nocturnal sleep disturbance. While nocturnal sleep disturbance was unrelated to motor symptom severity, it was, however, related to depressive symptoms, which accounted for around one-third of the variance. More significant nocturnal sleep disturbance was also related to greater l -dopa dose equivalents; however, the small sample size in this study precluded any attempt to discern whether these effects may be specifically related to dopamine agonist, l -dopa, or the combination of these medications. Overall, these findings are consistent with research linking depression and sleep disturbance 18 and support the notion that both features are likely to represent an underlying pathological correlate of the disease. They additionally suggest that daytime sleepiness in Parkinson’s disease is less influenced by depressive symptoms or motor disease severity, although sleepiness tends to be associated with the degree of nocturnal disturbance.
Although multivariate models should ideally include much larger sample sizes than those studied here, the key study findings presented are consistent with those published previously with much larger samples. 3 , 21 Additionally, these findings show that depressive symptoms and disease severity together predict over two-thirds of the variance in quality of life scores. While there was a unique contribution of depressive symptoms, motor disease severity, and sleep disturbance, depression was the largest independent predictor, uniquely accounting for around 21% of variance in quality of life scores. Additionally, a large proportion of the variance was shared, suggesting that these features are not operating independently. Overall, these findings are consistent with other reports using SCOPA-S which have demonstrated that the association between quality of life and sleep disturbance is only low to moderate and that depression and anxiety contribute to quality of life to a much greater extent than disease severity. 22 Interestingly, these relationships were apparent despite the fact that patients comprising this sample predominantly had rather mild to moderate disease severity, mild levels of depressive symptoms, and only mild levels of sleep disturbance.
The finding that nocturnal sleep disturbance is a unique predictor of quality of life as well as daytime sleepiness is important in terms of informing management approaches. As previously mentioned, sleep disturbance occurs in around two-thirds of Parkinson’s disease patients. 13 It may also be a risk factor for depression, cognitive decline, and dementia. 11 , 15 , 18 Recent work has demonstrated that more continuous dopaminergic stimulation with long-acting, extended-release dopamine agonists can improve nocturnal symptoms in Parkinson’s disease. 30 Furthermore, such agents may also offer improvements in the amelioration of depression. 30 While the etiological underpinnings of sleep disturbance and depression in Parkinson’s disease are unknown, it is possible that they reflect the involvement of common neurobiological pathways that are relevant for both sleep and mood. Further research examining sleep-wake disturbance in Parkinson’s disease is now required with specific reference to more detailed features such as REM sleep behavior disorder, insomnia, and circadian disturbance. In this regard, it is possible that the newer antidepressant agents that concurrently target the circadian system (i.e., melatonin) may be of benefit. Additional studies are now required to determine whether the aggressive treatment of depressive symptoms and sleep disturbance in Parkinson’s disease may offer significant benefit in terms of quality of life.
Importantly, the current data presented suggests that patients with Parkinson’s disease should be carefully assessed for depressive symptoms, which, even when mild, are an important and major contributor to both quality of life and sleep disturbance. Future research could aim to elucidate the commonalities between sleep disturbance and depression with a view to informing management. Distinct patterns of disease phenotype characterized by motor and nonmotor features have been well described in Parkinson’s disease and are likely to represent underlying patterns of neuropathology. 1 , 31 The shared pattern of depression and sleep disturbance may relate to particular subtypes of Parkinson’s disease, and prior research 1 suggests that such symptoms are likely to be evident in those with the nontremor dominant subtype.
This study could have been enhanced by the inclusion of a larger sample size. Additionally, since disease severity in this sample was mild to moderate, it is possible that it may not be representative of the larger Parkinson’s disease population. Given that sleep disturbance, depression, and quality of life each encompass a variety of domains (e.g., insomnia, REM sleep behavior disorder, apathy, loss of motivation, social isolation, physical disability), it is difficult in a small clinical study to dissect these complex interrelated multifactorial relationships. In this regard, larger studies in well-characterized patient cohorts that implement Parkinson’s disease-specific assessment tools are now required to evaluate these issues of disease heterogeneity.
1. Lewis SJG, Foltynie T, Blackwell AD, et al: Heterogeneity of Parkinson’s disease in the early clinical stages using a data-driven approach. J Neurol Neurosurg Psychiatry 2005; 76:343–348Google Scholar
2. Aarsland D, Larsen JP, Tandberg E, et al: Predictors of nursing home placement in Parkinson’s disease: a population-based prospective study. J Am Geriatr Soc 2000; 48:938–942Google Scholar
3. McKinlay A, Grace RC, Dalrymple-Alford JC, et al: A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism Relat Disord 2008; 14:37–42Google Scholar
4. Hely MA, Reid WG, Adena MA, et al: The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23:837–844Google Scholar
5. Naismith SL, Glozier N, Burke D, et al: Early intervention for cognitive decline: is there a role for multiple medical or behavioral interventions? Early Interv Psychiatry 2009; 3:19–27Google Scholar
6. Hobson P, Meara J: Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord 2004; 19:1043–1049Google Scholar
7. Aarsland D, Bronnick K, Ehrt U, et al: Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated caregiver stress. J Neurol Neurosurg Psychiatry 2007; 78:36–42Google Scholar
8. Gjerstad MD, Aarsland D, Larsen JP: Development of daytime somnolence over time in Parkinson’s disease. Neurology 2002; 58:1544–1546Google Scholar
9. Santamaria J, Tolosa E, Valles A: Parkinson’s disease with depression: a possible subgroup of idiopathic parkinsonism. Neurology 1986; 36:1130–1133Google Scholar
10. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al: Predictors and course of health-related quality of life in Parkinson’s disease. Mov Disord 2008; 23:1420–1427Google Scholar
11. Marion MH, Qurashi M, Marshall G, et al: Is REM sleep behavior disorder (RBD) a risk factor of dementia in idiopathic Parkinson’s disease? J Neurol 2008; 255:192–196Google Scholar
12. Slaughter JR, Slaughter KA, Nichols D, et al: Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001; 13:187–196Google Scholar
13. Garcia-Borreguero D, Larrosa O, Bravo M: Parkinson’s disease and sleep. Sleep Med Rev 2003; 7:115–129Google Scholar
14. De Cock VC, Vidailhet M, Arnulf I: Sleep disturbances in patients with parkinsonism. Nat Clin Pract Neurol 2008; 4:254–266Google Scholar
15. Naismith SL, Norrie L, Lewis SJ, et al: Does sleep disturbance mediate neuropsychological functioning in older people with depression? J Affect Disord 2009; 116:139–143Google Scholar
16. Dew MA, Hoch CC, Buysse DJ, et al: Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med 2003; 65:63–73Google Scholar
17. Geerlings SW, Beekman AT, Deeg DJ, et al: Duration and severity of depression predict mortality in older adults in the community. Psychol Med 2002; 32:609–618Google Scholar
18. Cho HJ, Lavretsky H, Olmstead R, et al: Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry 2008; 165:1543–1550Google Scholar
19. Ravina B, Elm J, Camicioli R, et al: The course of depressive symptoms in early Parkinson’s disease. Mov Disord 2009; 24:1306–1311Google Scholar
20. Cole SA, Woodard JL, Juncos JL, et al: Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996; 8:20–25Google Scholar
21. Schrag A, Jahanshahi M, Quinn N: What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 69:308–312Google Scholar
22. Martinez-Martin P, Visser M, Rodriguez-Blazquez C, et al: SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson’s disease. Mov Disord 2008; 23:1681–1688Google Scholar
23. Fahn S, Elton R, Marsden CD, et al: Unified Parkinson’s Disease Rating Scale: Recent Developments in Parkinson’s Disease. New Jersey, Macmillan Health Care Information, 1987, pp 153–163Google Scholar
24. Katzenschlager R, Head J, Schrag A, et al: Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in Parkinson’s disease. Neurology 2008; 71:474–480Google Scholar
25. Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory—II. San Antonio, Tex, Psychological Corp, 1996Google Scholar
26. Schrag A, Barone P, Brown RG, et al: Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord 2007; 22:1077–1092Google Scholar
27. Marinus J, Visser M, van Hilten JJ, et al: Assessment of sleep and sleepiness in Parkinson disease. Sleep 2003; 26:1049–1054Google Scholar
28. Peto V, Jenkinson C, Fitzpatrick R: PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol 1998; 245(suppl 1):S10–14Google Scholar
29. Hagell P, Nygren C: The 39-item Parkinson’s disease questionnaire (PDQ-39) revisited: implications for evidence-based medicine. J Neurol Neurosurg Psychiatry 2007; 78:1191–1198Google Scholar
30. Pahwa R, Stacy MA, Factor SA, et al: Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology 2007; 68:1108–1115Google Scholar
31. Lewis SJ, Barker RA: Understanding the dopaminergic deficits in Parkinson’s disease: insights into disease heterogeneity. J Clin Neurosci 2009; 16:620–625Google Scholar



