Patient With Voltage-Gated Potassium-Channel (VGKC) Limbic Encephalitis Found to Have Creutzfeldt-Jakob Disease (CJD) at Autopsy
To the Editor: Key differential diagnoses of patients presenting with rapidly-progressive dementia include autoimmune encephalitis, such as antibodies to the voltage-gated potassium channel (VGKC), and Creutzfeldt-Jakob disease (CJD). Correct and timely diagnosis of the etiology is paramount, given the catastrophic progression of CJD and the potential for response to immunomodulatory therapy in autoimmune encephalitis.
The antemortem diagnosis of rapidly-progressive dementia is predominantly clinical. For example, CJD has established diagnostic criteria that include clinical examination, characteristic findings on electroencephalography (EEG) and brain magnetic resonance imaging (MRI), and cerebrospinal fluid (CSF) 14–3−3.1,2 This is in contrast to VGKC limbic encephalitis, which does not have established diagnostic criteria. VGKC typically presents with confusion, seizures, psychosis, and hyponatremia.3 An underlying cancer has been identified in 47% of patients with VGKC.3
CJD has been previously promoted as being a mimic of VGKC limbic encephalitis.4 However, the significance of VGKC antibodies in patients with strong clinical, serologic, and/or imaging evidence of CJD is uncertain. We present a patient with rapidly-progressive dementia who was positive for the VGKC antibody, but with histopathological diagnosis of prion disease at autopsy.
METHODS
One case was retrospectively identified between 2009 and 2010 at a tertiary-care referral center. The clinical history, laboratories, imaging, and therapies were reviewed.
RESULTS
Case Report
A 64-year-old woman presented with a 17-month history of progressive gait instability and confusion. On admission, she had a brain MRI that showed restricted diffusion in the basal ganglia/thalamus and cerebral cortex, including the parietal, occipital, and frontal regions (Figure 1). Her lumbar puncture had an elevated tau biomarker (1,979 pg/mL; diagnostic cut-off: >1,200 pg/mL) and positive 14–3−3 in the CSF. Her serum VGKC antibody titer was elevated, at 0.17 nmol/L (normal: ≤0.02 nmol/mL). Imunomodulatory therapy with intravenous immunoglobulin was discussed, but the patient expired before treatment. Immunopathology showed spongiform degeneration, consisting of large vacuoles on hematoxylin-eosin staining. Misfolded prion protein (PrP) and kuru plaques were identified on immunohistochemistry, suggestive of sporadic CJD (Figure 2). Molecular subtype was MV1&2, as per the criteria developed by Parchi and colleagues.5
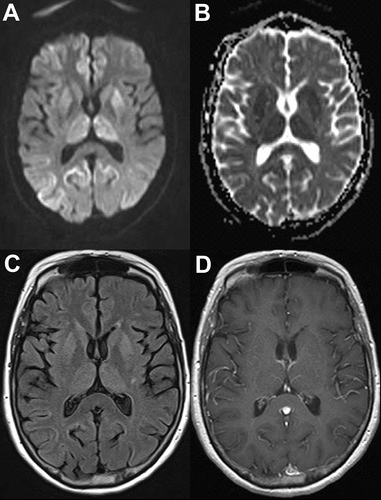
The hyperintensity can also be seen on fluid-attenuated inversion recovery sequence (FLAIR) [C]. There is no gadolinium enhancement [D]. These sequences are suggestive of Creutzfeldt-Jakob disease.
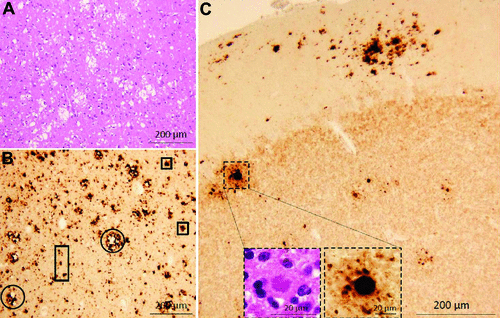
Discussion
Our findings illustrate the clinical presentation of progressive dementia with positive serology for VGKC antibodies (0.17 nml/L) in a patient who was later identified to have CJD at autopsy. The elevation of VGKC antibody in our patient is considered significant, since previous studies have discussed the significance of positive VGKC titer being >100 pmol/L.6,7 However, the threshold of positive serum antibodies varies between differing laboratories.
The significance of autoimmune limbic encephalitis mimicking CJD has been previously discussed in 15 cases.4 In this case series, patients were referred for possible CJD, based on examination findings. However, on immunfluorescence screening, VGKC antibodies were incidentally found. None of these patients met criteria for CJD, and in those who had autopsy, none had CJD.
Even though VGKC antibodies might be incidentally found in limbic encephalitis, the overlap observation of VGKC and CJD that we present is significant. Any rapidly-progressive dementia not responding to immunomodulation should have further diagnostic work-up for prion disease, despite positive antibody testing. Repeating lumbar puncture for CSF biomarkers, brain MRI, or EEG and histopathological diagnosis may be warranted. The significance of VGKC antibodies in patients with histopathologically-confirmed prion disease may be a reflection of this nonimmune-mediated neuronal degeneration, and it warrants further exploration.
1
2 : Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668Crossref, Medline, Google Scholar
3 : Clinical spectrum of voltage-gated potassium-channel autoimmunity. Neurology 2008; 70:1883–1890Crossref, Medline, Google Scholar
4 : Voltage-gated potassium-channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol 2008; 65:1341–1346Crossref, Medline, Google Scholar
5 : Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46:224–233Crossref, Medline, Google Scholar
6 : Voltage-gated potassium-channel antibodies in limbic encephalitis. Ann Neurol 2003; 54:530–533Crossref, Medline, Google Scholar
7 : Voltage-gated potassium-channel antibody-mediated syndromes: a spectrum of clinical manifestations. Rev Neurol Dis 2008; 5:65–72Medline, Google Scholar



