MM1-Type Sporadic Creutzfeldt-Jakob Disease With Early Behavioral Changes and Prolonged Symptom Duration
To the Editor: Creutzfeldt-Jakob disease (CJD) is a fatal neurodegenerative prion disease, most commonly presenting with memory loss, aphasia, and frontal/executive dysfunction.1,2 Other presenting symptoms include cerebellar dysfunction, constitutional symptoms, or behavioral changes and psychiatric symptoms.1 Eighty-five percent of CJD cases are sporadic (sCJD), resulting from spontaneous transformation of the prion protein into a misfolded conformation.3 The duration of symptoms of sCJD is variable and is related, in part, to the different molecular subtypes of the prion protein.4 Parchi et al.4,5 classified six main subtypes of sCJD based on a methionine/valine polymorphism at codon 129 of the prion protein gene (PRNP) and the associated type of prion protein (1 or 2). Classification of sCJD based on genotype and molecular markers allows for characterization of the associated phenotypes, including symptoms at disease onset, age at onset, and duration of symptoms. We report a case of MM1-type sCJD with a symptom duration of at least 48 months.
Case Report
A 61-year-old Caucasian man presented with 4 years of cognitive decline and behavioral changes. He was previously a high-functioning attorney who held statewide honors and leadership positions in his field. Four years before admission, he began implementing unethical billing practices, a sharp divergence from his previous behavior. After falsely accusing his clients regarding their billing, he faced lawsuits that he subsequently lost. This led to closure of his practice and reprimands from his professional organization for ethics violations. His poor business decisions eventually led to the foreclosure of his home. One year before presentation, he exhibited apathy and decreased attention to hygiene. For the next several months, he exhibited paranoia and aggressive behavior. This included a delusion that spies from foreign governments were watching him. By the time his wife sought medical attention, the patient’s memory was impaired and he could not remember that he was married. His symptoms worsened dramatically 1 month before presentation, when he became fixated on an impending terrorist attack around the anniversary of Sept. 11, 2001. He expressed visual and auditory hallucinations and was ultimately admitted to a psychiatric hospital for bizarre and aggressive behavior. After neurological evaluation and brain imaging, the patient was transferred to our hospital for further evaluation. Upon arrival at our facility, his neurological examination was notable for inattention, echolalia, and inability to follow commands. His motor examination showed that he had symmetrically increased tone and occasional asynchronous myoclonic jerks in his bilateral upper extremities. Reflex testing showed ankle clonus bilaterally. The patient had a slow, broad-based gait. MRI of the brain with and without contrast showed restricted diffusion in the posterior parietal cortices (Figure 1 [A]). A CSF paraneoplastic autoantibody panel was negative. Levels of 14-3-3 protein were not elevated; however, neuron-specific enolase levels were elevated at 46 ng/mL. A right frontal brain biopsy revealed spongiotic features, and immunoblot staining was positive for protease-resistant prion protein PrP 27–30 (Figure 1 [B]). Treatment with atypical antipsychotics and benzodiazepines for aggression and agitation provided minimal benefit, and the patient’s neurological symptoms worsened during his hospitalization. The patient ultimately died while awaiting placement in a nursing facility. Autopsy confirmed the diagnosis of prion disease with characteristics of sCJD. Molecular testing revealed methionine homozygosity at codon 129 of PRNP and type 1 protease-resistant prion protein (MM1).
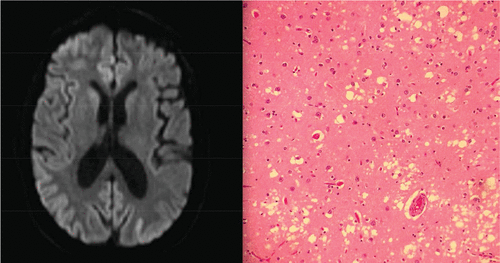
FIGURE 1. MRI Scan of the Brain With Diffuse Gyral High Diffusion–Weighted Signal Abnormality, Greatest in the Bilateral Parietal Gyri [A], and Microphotograph of the Frontal Cortex Demonstrating Spongiform Changes With Vacuolization and Neuronal Loss [B].
Discussion
In approximately one of four cases of sCJD, the presenting symptom is psychiatric or behavioral.2 As discussed by Ali et al.,2 it is not uncommon for patients with sCJD to be initially misdiagnosed with a primary psychiatric disease and admitted to a psychiatric unit, as was the case with our patient. His history of poor financial and legal decisions is consistent with the executive dysfunction, poor judgment, and impulsive behavior seen in sCJD. Given the success our patient achieved in his career, his poor decision making resulted in lawsuits brought against him and subsequent disbarment.
Our patient’s prolonged course of at least 48 months is atypical for sCJD. Of those with sCJD, 85% die within 12 months of symptoms.3 Brown et al.,6 in their consecutive series of 230 patients with neuropathologically verified CJD, reported only five patients with duration of symptoms longer than 4 years. In this series, the median duration of symptoms was 4 months and the mean duration was 7.6 months; 90% of patients died within 1 year of symptom onset.6 Our patient’s symptom duration is even more notable when considering his sCJD molecular subtype, MM1. Parchi et al.4 originally published 186 cases of MM1- and MV1-type sCJD with a mean symptom duration of 3.9 months and a range of 1–18 months. MM1 and MV1 were combined, given their similar pathological features.7 In their 2009 series, Parchi et al.5 reported 66 cases of MM1- and MV1-type sCJD with a mean symptom duration of 4.0 months and a range of 1–24 months. Subtypes other than MM1 are associated with significantly longer duration. The longest mean symptom duration by subtype reported by Parchi et al.5 was 20.0 months, associated with the MM/MV 2C subtype. Thus, we report the longest duration of symptoms for sCJD associated with the MM1 subtype.
The variable and oftentimes subtle first manifestations of the disease may limit the establishment of a clear onset of symptoms and thus an accurate duration of symptoms. Our patient’s profession provided opportunities for early behavioral changes to manifest themselves in legal and financial difficulties. This allowed symptom onset to be more easily established. In addition to molecular subtype, reasons for variable duration of symptoms in sCJD are unclear. One consideration in this case is that our patient’s age and educational attainment may have influenced his symptom duration via cognitive reserve, although this has not been documented in sCJD. That other genetic factors may influence symptom duration is supported by previously reported racial differences.8,9 Furthermore, both new variant CJD, a relatively new phenotype of CJD, and familial prion diseases are commonly associated with longer symptom duration than sCJD.7,10 This case suggests the importance of factors other than molecular subtype, whether environmental, demographic, or other genetic modifiers, in determining sCJD symptom duration.
1 : First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287Crossref, Medline, Google Scholar
2 : Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci 2013; 25:335–338Link, Google Scholar
3 : Infections of the nervous system: prion diseases, in Bradley’s Neurology in Clinical Practice, 1st ed. Edited by Daroff RB, Bradley WG. Philadelphia, Elsevier/Saunders, 2012, pp 1268–1282Crossref, Google Scholar
4 : Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46:224–233Crossref, Medline, Google Scholar
5 : Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol 2009; 118:659–671Crossref, Medline, Google Scholar
6 : Creutzfeldt-Jakob disease: clinical analysis of a consecutive series of 230 neuropathologically verified cases. Ann Neurol 1986; 20:597–602Crossref, Medline, Google Scholar
7 : Sporadic and familial CJD: classification and characterisation. Br Med Bull 2003; 66:213–239Crossref, Medline, Google Scholar
8 : Duration of prion disease is longer in Japan than in other countries. J Epidemiol 2011; 21:255–262Crossref, Medline, Google Scholar
9 : Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol 1979; 5:177–188Crossref, Medline, Google Scholar
10 : Diagnosis of new variant Creutzfeldt-Jakob disease. Ann Neurol 2000; 47:575–582Crossref, Medline, Google Scholar



