Poststroke Depression: Contributions From Network Science
Worldwide, stroke is the third most common cause of disability.10 Although the mortality rate and incidence of stroke has decreased in much of the world, the overall stroke burden has increased.11,12 Most worrisome, the rates of stroke in younger adults (20–64 years of age) may actually be increasing.10 Stroke-related disability burden is considerable due to long-term physical, cognitive, and psychological sequelae. Development of depression is one of the more common sequelae and is associated with multiple detrimental outcomes, making both prevention and prompt identification and treatment high priorities.13,14
A recent meta-analysis found that within the initial 5 years after a stroke, 31% of patients developed poststroke depression (PSD) (Figure 1).2 A similar prevalence (34%) of PSD was reported in a recent study at 2–5 years poststroke.15 A longitudinal study found that one-third of cases of PSD developed within the first 3 months after stroke, and half of these remitted by 1 year.1 A similar pattern was reported in a study of first-ever ischemic strokes (mostly mild) (Figure 1).3 It is noteworthy that two studies using data from stroke registries that included patients with transient ischemic attacks (TIAs) reported similar rates of depression following stroke and TIA (Figure 1).4,16 Relatively similar rates were also reported in a study limited to patients that were younger (18–50 years of age) at onset.17 Overall, these studies indicate that severity of brain injury is not a primary pathophysiological mechanism for PSD. This parallels what has been reported on development of major depression (MDD) after traumatic brain injury (Figure 1).5,18 In addition to psychological morbidity, PSD is also independently associated with more detrimental functional outcomes.13,19
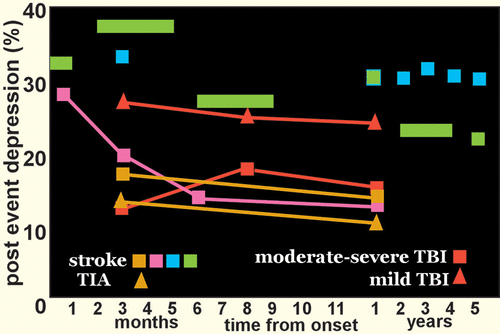
FIGURE 1. Poststroke depression (PSD) is common among survivors of stroke. A recent meta-analysis of population-based studies (green squares) and a longitudinal study that utilized a large stroke registry (blue squares) both reported that more than 30% of patients developed PSD within the first few months after a stroke.1,2 The longitudinal study also found that half of these cases remitted by 1 year.1 A similar pattern was reported in a study of first-ever ischemic stroke (pink squares).3 Of note, a study using data from a stroke registry that included patients with transient ischemic attacks (TIAs) reported similar rates of depression following stroke (gold squares)and TIA (gold triangles).4 Rates of developing major depression (MDD) after traumatic brain injury (TBI) are also similar when patients with moderate-severe TBI (orange squares) are compared with patients with mild TBI (orange triangles).5 Overall, these results suggest that severity of brain injury is not a primary pathophysiological mechanism for development of depression.
There is considerable debate regarding the effect of lesion location on risk of PSD. Two meta-analyses failed to find support for a relationship between lesion location and PSD.20,21 Another reported a very weak correlation between right hemisphere lesions and PSD.22 In contrast, two later meta-analyses reported slightly increased frequency of PSD with left hemisphere lesions, but in one study this was specific to the acute phase and in the other study it was specific to the subacute phase.23,24 A smaller meta-analysis found an inverse correlation between severity of depression and distance from lesion to the frontal pole, but only for left hemisphere strokes.25 Common methodological weaknesses identified in these studies include usage of many different instruments for identification of PSD, considerable range in time from event, visual analysis of lesion locations, and the systematic exclusion of patients with language dysfunction.26
Recent studies utilizing more advanced approaches to lesion-symptom mapping (e.g., statistical parametric mapping, multivariate lesion-symptom mapping) have not resolved the debate. In one study, injury to left putamen, right insula and right superior longitudinal fasciculus were associated with presence of major depression (Hamilton Rating Scale for Depression-24 [HAM-D-24] score ≥20) at 1 month.27 In another, higher symptoms of depression (Hospital Anxiety and Depression Scale) in the acute-subacute stage correlated with injury to brainstem, left basal ganglia, and left ventrolateral prefrontal cortex.28 In contrast, the study that focused on left hemisphere strokes in the chronic stage reported that greater symptoms of depression (Stoke Aphasic Depression Questionnaire) were associated with injury to left dorsolateral prefrontal cortex.29 A fourth study reported that no areas were significantly associated with a diagnosis of PSD once correction for multiple comparisons was done.30 Other recent studies have also reported a lack of association between either stroke location or volume and presence of PSD, indicating the importance of other factors.6,8,31 As with other areas of PSD study, this remains an active area of inquiry.
Although there may not be a definitive relationship between lesion location and PSD, another growing area of research is alterations to non-lesion anatomical locations and alterations of connectivity networks outside of the discrete lesion. Recent studies have focused upon understanding the role of altered networks, determined by either structural or functional connectivity, especially in the setting of recovery from loss of brain parenchyma, as in stroke.32–35 Alteration of the most richly connected areas (“hubs”) of the connectome have been implicated in numerous brain disorders, including stroke.36–38 Structural (anatomic) connectivity is commonly assessed using diffusion tensor imaging (DTI) tractography based metrics that estimate strength of physical connections between areas (nodes).39,40 Functional connectivity is commonly assessed using resting-state functional MRI (rs fMRI) metrics that are based on temporal correlation of spontaneous signal intensity changes within two areas (e.g., voxel, region of interest).32,39–41 Network structure is most altered in the acute to subacute period following stroke.33,34 Normalization of network structure has been shown to parallel recovery in the domains of language, memory and attention.34
Studies comparing connectivity metrics between stroke patients with and without PSD are considered the most useful for identifying symptom-related network changes.42 A rs fMRI study that utilized bilateral seeds (posterior cingulate/retrosplenial, anterior cingulate and dorsolateral prefrontal cortices) to assess functional connectivity in three networks (chosen because they have been implicated in MDD) in the early subacute (<2 weeks) period reported multiple areas in which stroke patients with and without PSD differed (Figure 2).7 Another rs fMRI study in the subacute (<1 month) stage also reported higher functional connectivity between posterior cingulate/retrosplenial cortex and inferior parietal cortex in stroke patients with PSD compared with stroke patients without PSD (Figure 2).6 A rs fMRI study in the early chronic (3 months) stage that utilized the amplitude of low frequency fluctuations (ALFF) to assess regional activity level reported higher fractional ALFF (fALFF) in the left dorsolateral prefrontal cortex and right precentral gyrus in patients with PSD compared with patients without PSD.31 One source of variance in these studies is that their groups encompassed all stroke locations. The other chronic stage (3–12 months) rs fMRI study compared patients with and without PSD grouped by stroke location (frontal, parietal, temporal).8 This study assessed whole brain functional connectivity at the voxel level (degree centrality, number of connections). Of note, stroke location affected where differences in degree centrality between patients with and without PSD were located and in some instances the direction of differences (Figure 2).8
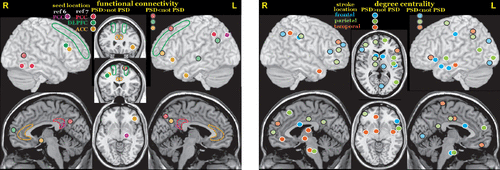
FIGURE 2. Several studies have compared resting-state functional MRI (rs fMRI) functional connectivity between stroke patients with and without PSD to identify network changes that might be symptom related. Left. Two studies utilized bilateral seeds (outlined areas) to assess functional connectivity in the early subacute stage.6,7 Color-coded solid circles indicate areas in which connectivity differed between the groups. Both studies combined across stroke locations. Right. A study in the chronic stage poststroke (3–12 months) compared patients with and without PSD grouped by stroke location (frontal, parietal, temporal).8 This study assessed whole-brain functional connectivity at the voxel level (degree centrality, number of connections). Of note, stroke location affected where differences in degree centrality between patients with and without PSD were located and in some instances the direction of differences.
A few studies have assessed relationships between connectivity metrics and severity of depression symptoms. A DTI study compared structural connectivity (node degree) of 90 nodes (regions of interest) derived by using the automated anatomic labeling (AAL) atlas to parcellate the brain.27 Lower connectivity at one week correlated with higher severity of depression (HAM-D-24) at 1 month for 17 of the nodes (bilateral - superior frontal gyrus, posterior cingulate gyrus, fusiform gyrus, insula, caudate; left: inferior frontal gyrus, superior temporal gryus, olfactory cortex, precuneus; right: precentral gyrus, putamen).27 Both rs fMRI studies that utilized bilateral seeds reported that severity of depression (HAM-D-17; Beck Depression Inventory, BDI) in the PSD group was significantly positively correlated with increased functional connectivity between posterior cingulate/retrosplenial cortex (default mode network) and inferior parietal cortex (Figure 3 and cover).6,7 The study that assessed other seeds reported that the severity of depression (HAM-D-17) in the PSD group was also positively correlated with increased functional connectivity of the anterior cingulate cortex (affective network) to orbital inferior frontal gyrus, with a trend negative correlation with decreased functional connectivity of dorsolateral prefrontal cortex (cognitive control network) to angular gyrus (Figure 3 and cover).7 In a set of rs fMRI studies in the early chronic stage (3 months) after mostly minor strokes, severity of depression symptoms (Patient Health Questionnaire, PHQ-9) correlated with higher fALFF in the left insula/superior temporal gyrus and with lower seed-based functional connectivity of the left dorsolateral prefrontal cortex to right supramarginal gyrus (Figure 3).9,31 Another rs fMRI study that evaluated functional connectivity between all voxels and the default mode network (identified by independent component analysis) reported functional connectivity at ten days correlated with symptoms of depression (HAM-D-17) at 3 months (but not at ten days) for three areas.43 The correlation was positive for middle temporal cortex and precuneus, negative for neostriatum. As noted in many of these studies, these network changes are generally consistent with what has been reported for MDD.41,44,45
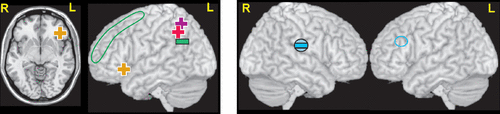
FIGURE 3 AND COVER. Left and Cover. Although there were many areas where seed based functional connectivity differed between patients with and without PSD in the rs fMRI studies detailed above, only a few correlated with severity of depressive symptoms. Both studies reported that increased functional connectivity between the seeds in the posterior cingulate cortex (default mode network) and left parietal cortex (pink plus sign, purple plus sign) positively correlated with severity of depression.6,7 One study also reported that severity of depression was positively correlated with increased functional connectivity between the anterior cingulate cortex seeds (affective network) and left orbital inferior frontal gyrus (gold plus signs), and a trend negative correlation with reduced connectivity between the dorsolateral prefrontal cortex seeds (cognitive control network) and left angular gyrus (green minus sign).7 Right: In contrast, a set of rs fMRI studies in the early chronic stage (3 months) reported that severity of depression correlated with lower functional connectivity between the seed in left dorsolateral prefrontal cortex (blue circle) and right supramarginal gyrus (blue filled circle and negative sign).9
Despite the consensus regarding the prevalence and burden of PSD, there is substantial disagreement regarding the pathophysiology. Proposed biological contributors to PSD include alterations in neurotrophic factors, hypothalamic-pituitary-adrenal axis dysfunction, the inflammatory cascade and cell-mediated immune activation that follows stroke.13,46–48 A study that correlated the proteomic profile with depressive symptoms at 3 months poststroke supported the presence of peripheral immunodepression due to neuroinflammatory processes.49 The interaction of the inflammatory response with neurotrophic factors has become a promising avenue of study. The most important neurotrophin implicated thus far is brain-derived neurotrophic factor (BDNF), shown as early as 1995 to promote neuronal survival in vitro.50 Low circulating BDNF levels have been associated with an increased risk of stroke.51 Low levels of BDNF in the acute stage of stroke have been associated with poor functional outcomes at two-year and seven-year follow ups, independent of stroke severity.52 A recent meta-analysis found that peripheral BDNF levels in the acute stage were lower in stroke patients who went on to develop PSD, suggesting potential as a biomarker.53 Although a connection between polymorphisms in BDNF genotype and a propensity for PSD has been reported in some studies, a recent meta-analysis found a relationship to significantly lower risk of ischemic stroke, but no role in PSD.54 Many of these factors have also been implicated in MDD.46,55–57 For example, meta-analyses indicate elevations of multiple peripheral biomarkers of inflammation (e.g., cytokines, interleukins, chemokines) in MDD, as well as decreased peripheral levels of some immune mediators following treatment with antidepressants.58–62 A recent meta-analysis that focused on BDNF found support for a link between polymorphisms in BDNF genotype and risk of late life depression, decreased peripheral levels of BDNF in patients with MDD, and increases in BDNF following treatment with selective serotonin reuptake inhibitors (SSRIs) or electroconvulsive therapy.63 It is noteworthy serotonin-norepinephrine reuptake inhibitors did not increase BDNF levels.
Although multiple treatment modalities have been studied, there is considerable heterogeneity in key methodological aspects such as time from stroke and duration of treatment. Several meta-analyses of randomized control trials have determined that antidepressants were better than placebo for improving symptoms of depression in patients with PSD, although medications varied considerably in both efficacy and tolerability.64–67 The potential of antidepressants to prevent development PSD in nondepressed stroke patients is also supported by several meta-analyses.64,68,69 In addition, there is growing evidence that treatment with SSRIs in particular can improve functional recovery (e.g., dependence, disability, neurological impairment) after stroke, although the meta-analysis did not address whether neurological recovery occurred independently of improvement in depression.65,70 A recent meta-analysis of randomized control trials in which SSRIs were initiated within the first month in stroke patients without PSD reported improved neurologic functioning (e.g., decreased disability, increased functional independence).71 Contrary to expectations, SSRIs did not decrease incidence of PSD. Animal studies indicate multiple possible mechanisms of action (e.g., promoting neurogenesis, increasing neurotrophins including BDNF, decreasing inflammation).70,72
Conclusions
Understanding of the prevalence, influence, and pathophysiology of PSD have advanced over the previous two decades. An important advance has been the shift from a primary focus on lesion location to study of brain functional and structural network changes after stroke. The study of PSD has also led to possible interventions that may facilitate motor and cognitive recovery. Emerging insights into the connectome, BDNF, and the role of the poststroke inflammatory cascade are leading to better understanding of PSD and may, in time, identify new prophylactic or therapeutic options.
1 : The natural history of depression up to 15 years after stroke: the South London Stroke Register. Stroke 2013; 44:1105–1110Crossref, Medline, Google Scholar
2 : Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014; 9:1017–1025Crossref, Medline, Google Scholar
3 : The relationship between frontal lobe lesions, course of post-stroke depression, and 1-year prognosis in patients with first-ever ischemic stroke. PLoS One 2014; 9:e100456Crossref, Google Scholar
4 : Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012; 43:1609–1616Crossref, Google Scholar
5 : Depression in the first year after traumatic brain injury. J Neurotrauma 2018; 35:1620–1629Crossref, Google Scholar
6 : Depression and anxiety symptoms are associated to disruption of default mode network in subacute ischemic stroke. Brain Imaging Behav 2017; 11:1571–1580Crossref, Google Scholar
7 : Altered functional connectivity in post-ischemic stroke depression: a resting-state functional magnetic resonance imaging study. Eur J Radiol 2018; 100:156–165Crossref, Google Scholar
8 : A study of the brain functional network of post-stroke depression in three different lesion locations. Sci Rep 2017; 7:14795Crossref, Google Scholar
9 : Lower cognitive control network connectivity in stroke participants with depressive features. Transl Psychiatry 2018; 7:4Crossref, Google Scholar
10 : Global burden of stroke. Circ Res 2017; 120:439–448Crossref, Google Scholar
11 :
12 :
13 :
14 : Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev 2018; 90:104–114Crossref, Google Scholar
15 : Poststroke depression: a long-term problem for stroke survivors. Am J Phys Med Rehabil 2018; 97:565–571Crossref, Google Scholar
16 : Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol 2014; 14:198Crossref, Google Scholar
17 : Long-term depressive symptoms and anxiety after transient ischaemic attack or ischaemic stroke in young adults. Eur J Neurol 2016; 23:1262–1268Crossref, Google Scholar
18 : Epidemiology and natural history of psychiatric disorders after TBI. J Neuropsychiatry Clin Neurosci (Epub ahead of print, June 25, 2018)Google Scholar
19 : Longitudinal impact of depression on quality of life in stroke patients. Psychiatry Investig 2018; 15:141–146Crossref, Google Scholar
20 : Depression after stroke and lesion location: a systematic review. Lancet 2000; 356:122–126Crossref, Medline, Google Scholar
21 : Post-stroke depression and lesion location: a systematic review. J Neurol 2015; 262:81–90Crossref, Medline, Google Scholar
22 : Relationship between post-stroke depression and lesion location: a meta-analysis. Kaohsiung J Med Sci 2004; 20:372–380Crossref, Medline, Google Scholar
23 : Imaging markers of post-stroke depression and apathy: a systematic review and meta-analysis. Neuropsychol Rev 2017; 27:202–219Crossref, Google Scholar
24 : The association between lesion location, sex and poststroke depression: meta-analysis. Brain Behav 2017; 7:e00788Crossref, Google Scholar
25 : A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci 2003; 15:422–430Link, Google Scholar
26 : Post-stroke depression: impact of lesion location and methodological limitations: a topical review. Front Neurol 2017; 8:498Crossref, Google Scholar
27 : A significant risk factor for poststroke depression: the depression-related subnetwork. J Psychiatry Neurosci 2015; 40:259–268Crossref, Medline, Google Scholar
28 : Neuroanatomic pathways associated with poststroke affective and apathetic depression. Am J Geriatr Psychiatry 2013; 21:840–847Crossref, Google Scholar
29 .: Depression symptoms in chronic left hemisphere stroke are related to dorsolateral prefrontal cortex damage. J Neuropsychiatry Clin Neurosci, 2016; 28:292–298Google Scholar
30 : Imaging predictors of poststroke depression: methodological factors in voxel-based analysis. BMJ Open 2014; 4:e004948Crossref, Medline, Google Scholar
31 : Fractional amplitude of low-frequency fluctuations (fALFF) in post-stroke depression. Neuroimage Clin 2017; 16:116–124Crossref, Google Scholar
32 : Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage 2012; 62:2271–2280Crossref, Google Scholar
33 : The connectomics of brain disorders. vol. 16. 2015, pp 159–172Google Scholar
34 : Re-emergence of modular brain networks in stroke recovery. Cortex 2018; 101:44–59Crossref, Google Scholar
35 : On the low dimensionality of behavioral deficits and alterations of brain network connectivity after focal injury. Cortex 2018; pii: S0010-9452(17)30425-2Crossref, Google Scholar
36 : Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci USA 2012; 109:20608–20613Crossref, Google Scholar
37 : The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014; 137:2382–2395Crossref, Medline, Google Scholar
38 : The “Hub Disruption Index”: a reliable index sensitive to the brain networks reorganization: a study of the contralesional hemisphere in stroke. Front Comput Neurosci 2016; 10:84Crossref, Google Scholar
39 : Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist 2015; 21:290–305Crossref, Google Scholar
40 : What we know about the brain structure-function relationship. Behav Sci (Basel) 2018; 8:E39Crossref, Medline, Google Scholar
41 : A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci Ther 2018; Epub ahead of printCrossref, Google Scholar
42 : Measuring functional connectivity in stroke: Approaches and considerations. J Cereb Blood Flow Metab 2017; 37:2665–2678Crossref, Google Scholar
43 : Subacute default mode network dysfunction in the prediction of post-stroke depression severity. Radiology 2012; 264:218–224Crossref, Medline, Google Scholar
44 : Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res 2017; 92:147–159Crossref, Google Scholar
45 : Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety 2017; 34:9–24Crossref, Medline, Google Scholar
46 : Inflammation and depression: why poststroke depression may be the norm and not the exception. Int J Stroke 2011; 6:128–135Crossref, Google Scholar
47 : Meta-analyses indicate associations between neuroendocrine activation, deactivation in neurotrophic and neuroimaging markers in depression after stroke. J Stroke Cerebrovasc Dis 2013; 22:e124–e135Crossref, Medline, Google Scholar
48 : Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther 2018; 184:131–144Crossref, Google Scholar
49 : A pathway proteomic profile of ischemic stroke survivors reveals innate immune dysfunction in association with mild symptoms of depression: a pilot study. Front Neurol 2016; 7:85Crossref, Google Scholar
50 : A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995; 374:450–453Crossref, Google Scholar
51 : Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke 2013; 44:2768–2775Crossref, Google Scholar
52 : Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke 2016; 47:1943–1945Crossref, Google Scholar
53 : Decreased serum brain-derived neurotrophic factor may indicate the development of poststroke depression in patients with acute ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis 2018; 27:709–715Crossref, Google Scholar
54 : Meta-analysis on the association between brain-derived neurotrophic factor polymorphism rs6265 and ischemic stroke, poststroke depression. J Stroke Cerebrovasc Dis 2018; 27:1599–1608Crossref, Google Scholar
55 : The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2016; 64:277–284Crossref, Google Scholar
56 : Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep 2017; 6:15–20Crossref, Google Scholar
57 : The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J Inflamm Res 2018; 11:179–192Crossref, Google Scholar
58 : A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457Crossref, Medline, Google Scholar
59 : Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49:206–215Crossref, Google Scholar
60 : A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2016; 68:1–8Crossref, Google Scholar
61 : A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016; 21:1696–1709Crossref, Medline, Google Scholar
62 : Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 2018; 55:4195–4206Google Scholar
63 : Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psychiatry 2018; 8:308Crossref, Google Scholar
64 : Interventions for preventing depression after stroke. Cochrane Database Syst Rev 2008; (3):CD003689Google Scholar
65 : Selective serotonin reuptake inhibitors for stroke recovery. JAMA 2013; 310:1066–1067Crossref, Medline, Google Scholar
66 : Efficacy and feasibility of antidepressant treatment in patients with post-stroke depression. Medicine (Baltimore) 2016; 95:e5349Crossref, Google Scholar
67 : Comparative efficacy and acceptability of antidepressant treatment in poststroke depression: a multiple-treatments meta-analysis. BMJ Open 2017; 7:e016499Crossref, Google Scholar
68 : Fluoxetine for the prophylaxis of poststroke depression in patients with stroke: a meta-analysis. Int J Clin Pract 2010; 64:1310–1317Crossref, Google Scholar
69 : Prevention of poststroke depression: does prophylactic pharmacotherapy work? J Stroke Cerebrovasc Dis 2013; 22:1243–1251Crossref, Medline, Google Scholar
70 : Selective serotonin reuptake inhibitors to improve outcome in acute ischemic stroke: possible mechanisms and clinical evidence. Brain Behav 2015; 5:e00373Crossref, Google Scholar
71 : Early selective serotonin reuptake inhibitors for recovery after stroke: a meta-analysis and trial sequential analysis. J Stroke Cerebrovasc Dis 2018; 27:1178–1189Crossref, Google Scholar
72 : Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci Rep 2017; 7:14926Crossref, Google Scholar



