Case Study 5: A 74-Year-Old Man With Dysphagia, Weakness, and Memory Loss
Case Presentation
A 74-year-old man presented for evaluation of swallowing difficulties, weakness, and memory concerns. The patient had been in his usual state of health until approximately 10 years prior to presentation, when at age 64, he first experienced difficulty swallowing. He underwent two dilatation procedures for a suspected esophageal stricture that failed to ameliorate the problem. Although he transitioned to eating a mechanical soft diet at home, he experienced coughing while eating and took several hours to finish a meal. He unintentionally lost approximately 30 pounds over the course of 2 years. Five years before presentation, he developed slowly progressive weakness in his hands and proximal legs, with notable difficulties standing from a seated position and ascending and descending stairs. Four years prior to this evaluation, he fell down the stairs at a train station, sustaining a small subarachnoid hemorrhage in the left posterior sylvian fissure and a cortical contusion within the right anterior temporal lobe. Over the prior 2 years, he had lost the ability to write due to finger weakness, required self-fashioned tools to open jars, transitioned to walking with a cane, and fell again at home when trying to load paper into his printer.
Over this same 2-year period, he experienced cognitive slowing, distractibility, difficulties retrieving words, and difficulties with memory of recent events and information. Others had noted that the patient would lose track of what exercises he had been doing when between sets at the gym and would repeat questions and statements more frequently than he had previously. As a recently retired professor of computer science, the patient had been engaged in research and supervising students until shortly before his evaluation. Cognitive changes had not contributed to his decision to retire or prevented him from engaging in any of his usual activities. When he and his wife applied for entry into a retirement community, his score on a cognitive screening test prompted referral for further assessment.
He had no history of somatosensory symptoms, pain, autonomic symptoms, shortness of breath, neuropsychiatric symptoms (including those involving mood, anxiety, psychosis, and personality changes), or sleep-related issues except for severe obstructive sleep apnea for which he was adherent with continuous positive airway pressure (CPAP) treatment. His medical and surgical histories also included hypertension, dyslipidemia, coronary artery disease, prostate cancer treated with prostatectomy, sensorineural hearing loss treated with hearing aids, cataracts, and right hip osteoarthritis treated with total hip replacement. His medications included aspirin, atenolol, and atorvastatin. His family history included a father who developed memory difficulties at age 74 and died the following year, but there was no family history of additional neurological illness, including neuromuscular disorders.
Questions: What are general diagnostic considerations regarding the motor and cognitive symptoms? How might a neurological examination help to refine the list of possible diagnoses?
An important consideration from the start is whether a single underlying condition accounts for both the cognitive and motor symptoms or whether separate conditions are responsible. The sequence of symptom development in which progressive motor symptoms developed years before the cognitive symptoms developed could also help identify a unifying condition that might have spread throughout the nervous system. These considerations require keeping in mind gradually progressive neurological disorders that are associated with cognitive and pyramidal or extrapyramidal motor symptoms and have insidious onset in adulthood (Table 1), while establishing the specific nature of the patient’s cognitive and motor changes by examination. Is the pattern of cognitive deficits suggestive of a neurodegenerative syndrome, or is it a relatively nonspecific pattern? Do the motor symptoms reflect true neuromuscular weakness, resulting from conditions involving the pyramidal pathway, neuromuscular junction, or muscles, or do the symptoms reflect elements of an extrapyramidal disorder that might affect fine motor dexterity, speed, or postural stability?
| Category | Examples | Cognitive or Behavioral Impairmentb | Motor Signsc | Testsd | Pathology |
|---|---|---|---|---|---|
| Neurodegenerative (extrapyramidal) | LBD, MSA, CBD, PSP, PiD, FXTAS, HD, WD | Attention, executive, language, social cognition, NPS | Parkinsonism, ataxia (MSA, FXTAS, WD), intention tremor (FXTAS), chorea (HD) | Brain MRI, FDG-PET, DAT scan, laboratory, genetics | Alpha-synuclein (LBD, MSA), tau (CBD, PSP, PiD), astrocytic inclusions (FXTAS), neuronal inclusions (HD) |
| Neurodegenerative (pyramidal) | FTD-MND | Executive, social cognition, language, NPS | UMN, LMN | Brain MRI, FDG-PET, EMG, genetics | TDP-43, FUS |
| Other neuromuscular | DM1 | Attention, executive | Skeletal muscle weakness, myotonia | EMG, genetics, brain MRI | Internal nuclei, type 1 myofiber atrophy, pyknotic nuclear clumps, ring fibers, sarcoplasmic masses |
| Vascular | SVD, CADASIL | Attention, executive | UMN, parkinsonism (variable) | Brain MRI, genetics | Arteriolar sclerosis, GOM (CADASIL) |
| Infectious | HAND or HAD, PML, GP | Attention, executive, visuospatial (PML), NPS (HAD, GP) | UMN, parkinsonism (variable) | Brain MRI, laboratory | Inflammation, demyelination, bizarre astrocytes, enlarged oligodendroglial nuclei (PML) |
| Autoimmune | PPMS | Attention, executive, memory, visuospatial | UMN, myelopathic signs | Brain MRI, laboratory | Inflammation, demyelination, oligodendroglial or axonal loss |
| Adult-onset leukodystrophies | X-ALD, MLD, Krabbe disease, ALSP | Attention, executive, NPS | UMN, parkinsonism, ataxia, myelopathic and neuropathic signs | Brain MRI, laboratory, genetics | Demyelination, axonal spheroids, pigmented glia (ALSP) |
| Structural | NPH, FBSS | Attention, executive, social cognition, NPS | UMN, parkinsonism (variable) | Brain MRI, CSF removal (NPH), spine MRI (FBSS) | Ventricular enlargement (NPH), absence of neurodegeneration |
TABLE 1. Conditions associated with adult-onset, gradually progressive cognitive and pyramidal or extrapyramidal motor dysfunctiona
This patient’s cognitive examination was notable for a score of 24 of a maximum 30 points on the Montreal Cognitive Assessment (scores ≤25 indicate a level of impairment), with points lost for two errors in the serial seven subtraction task and a score 0 of 5 points on a delayed word recall task (although all five words were identified with category cues). A neuropsychological evaluation completed prior to referral documented average performance on tests of visual and spatial functioning, average to below average performance on tests of processing speed and executive functioning, low average performance on tests of basic attention and semantic fluency (with average phonemic fluency and confrontation naming), and exceptionally low word-list learning. Specifically, on a 12-item word-list test, the patient acquired five, six, and five words over three trials and recalled two of the 12 words after a delay (recognition results are not reported).
Pertinent findings from the patient’s general neurological examination included a resting tremor of the jaw, with no additional tremors, masked facies, or bradykinesia; normal muscle tone and no percussion myotonia; presence of notable atrophy of the forearm flexor compartments and quadriceps bilaterally and mild proximal arm atrophy; no fasciculations; and diffuse, relatively symmetrical weakness that was most pronounced in the finger flexors and hip flexors. There were no pertinent somatosensory deficits. Deep tendon reflexes were trace throughout, and plantar responses were flexor. The patient walked with a stooped posture, assisted by a cane, and he was unable to walk on his heels or toes. The Romberg sign was not present.
Questions: How does information from the neuromuscular and cognitive examination help to narrow the differential diagnosis? What diagnostic tests and studies are indicated?
The most salient result from cognitive testing was the patient’s impaired word-list learning, particularly on the 12-item task with a list length that exceeds working memory span. Poor retention—computed as the number of words obtained on delayed recall divided by the maximum number of words obtained on any of the acquisition trials—suggests temporolimbic amnesia, which is in line with the reported history of difficulties remembering recent events and information (1). Taken together with borderline impairment in semantic fluency that was consistent with the reported history of difficulties retrieving words, this cognitive profile would most commonly be seen among those with Alzheimer disease (AD) (2).
The presence of neuromuscular weakness, atrophy, hyporeflexia, normal muscle tone, and no pertinent somatosensory deficits suggests a disorder affecting lower motor neurons or the muscles themselves. The absence of bradykinesia, rigidity, and hyperkinetic signs, such as limb tremor, dystonia, or dyskinesias, makes diseases involving the basal ganglia less likely. Conditions in the differential diagnosis include motor neuron disease (MND) and myopathies. The lack of fasciculations or accompanying signs of upper motor neuron dysfunction argues against MND. The distribution of prominent atrophy of the quadriceps and medial forearm flexors and weakness in wrist and finger flexion and knee extension in an older patient raises the possibility of sporadic inclusion body myositis (IBM), an inflammatory myopathy (3, 4).
Further workup is required to increase diagnostic confidence. Electromyography (EMG) and nerve conduction studies are indispensable for distinguishing between disorders of the motor neuron, nerve, neuromuscular junction, and muscle (5). Findings suggestive of MND would include evidence of widespread acute denervation (fasciculation potentials, fibrillations, and positive sharp waves), chronic reinnervation (motor unit potentials [MUPs] exhibiting increased amplitude, increased duration, and polyphasia), and a reduced recruitment pattern (reflecting a loss of motor units). Findings suggestive of myopathy would include polyphasic MUPs with decreased amplitude and duration and an early recruitment pattern (reflecting an unchanged number of motor units that are activated earlier than expected because the number of normally functioning muscle fibers is reduced). In the case of inflammatory myopathies, including IBM, EMG can also demonstrate “irritable” myopathic changes characterized by increased insertional and spontaneous activity with fibrillation potentials and positive sharp waves, although these findings are not specific (6).
A laboratory evaluation for muscle weakness should include a comprehensive metabolic panel and testing for calcium, magnesium, phosphate, creatine kinase, aldolase, lactate dehydrogenase, and thyroid-stimulating hormone levels (7). Given the possibility of IBM, testing for anticytosolic 5′-nucleotidase 1A (anti-NT5C1A) antibodies is recommended (8–10).
Additional recommended evaluation workup for cognitive dysfunction would include MRI of the brain and an assessment of vitamin B12 levels (11).
Results of the patient’s laboratory, diagnostic, and imaging tests are summarized in Box 1 and Figure 1.
BOX 1. Key findings from laboratory, diagnostic, and imaging tests of a 74-year-old patient with dysphagia, weakness, and memory loss
Electromyography (right arm and leg)
Increased spontaneous activity and early recruitment of short-duration small-amplitude polyphasic motor unit action potentials in most muscles studied.
Flexor digitorum profundus to digit 4: 2+ insertional activity, 2+ fibrillations, 2+ positive sharp waves, no fasciculations, no high frequency, −1 amplitude, −1 duration, 1+ polyphasia, and early recruitment pattern.
MRI (brain)
Moderate burden of T2 hyperintensities in the cerebral white matter.
Focus of susceptibility in the right anterior temporal cortex and multiple punctate foci of susceptibility in the left anterior temporal cortex.
Potential atrophy of the bilateral medial temporal, posterolateral temporal, dorsal frontal, and parietal cortices.
Laboratory tests
Creatine kinase level: 371 U/L (49−347 U/L, laboratory-specific reference range for healthy adult males).
Normal or unremarkable electrolytes, serum transaminases, aldolase, vitamin B12, and thyroid-stimulating hormone levels, complete blood count, and serum protein electrophoresis with immunofixation.
Nonreactive or normal HIV antibodies and antigens, hepatitis C virus antibodies, and Treponema pallidum immunoglobulin G or immunoglobulin M antibodies.
Antinuclear antibodies positive at 1:40, speckled pattern; normal anti-Ro (SS-A) and anti-La (SS-B) antibody titers; and negative anti-NT5C1A antibodies.
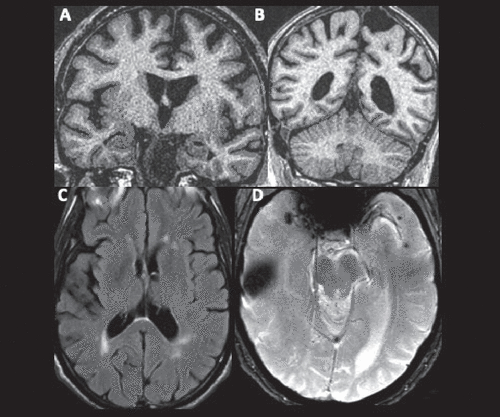
FIGURE 1. Brain MRI of a 74-year-old patient with dysphagia, weakness, and memory lossa
aA and B: Coronal high-resolution T1-weighted images reveal sulcal widening and increased width of the choroid fissure, suggesting mild to moderate atrophy in the posterolateral temporal, dorsal frontal, and parietal cortices and very mild atrophy in the medial temporal cortex. C: An axial fluid-attenuated inversion recovery image reveals T2 hyperintensities in the periventricular and subcortical white matter, suggesting microvascular ischemic disease. D: An axial T2* gradient echo image demonstrates a focus of susceptibility in the right anterior temporal cortex, suggesting prior contusion, and multiple punctate foci of susceptibility in the left anterior temporal cortex, suggesting microhemorrhages.
Questions: Considering the results of these tests, what initial diagnostic formulation would be appropriate? Is a parsimonious, single underlying condition likely?
The EMG findings of short-duration, low-amplitude MUPs with early recruitment suggest a myopathy rather than MND; increased insertional activity, fibrillation potentials, and positive sharp waves further suggest “irritable myopathy.” The mildly but not substantially elevated creatine kinase level argues against dermatomyositis and polymyositis, as does the lack of proximal arm weakness. Factoring in the patient’s age, the specific distribution of weakness, and the time course of insidious onset and gradual progression of symptoms, these EMG and laboratory results support a diagnosis of sporadic IBM. Although associated with sporadic IBM, anti-NT5C1A antibodies have reported diagnostic sensitivity for IBM, ranging from approximately 40% to 75% across studies (14, 15). However, their absence does not exclude the diagnosis, especially in the setting of an otherwise highly suggestive presentation.
A parsimonious, single cause of both the motor and cognitive symptoms appears less likely at this point. Mixed AD and cerebrovascular disease is not associated with focal muscle atrophy or myopathic EMG findings, even in later disease stages. Mutations in selected genes, including valosin-containing protein (VCP), cause hereditary multisystem proteinopathy (MSP) with heterogeneous phenotypes characterized by frontotemporal dementia (FTD), Paget disease of bone, myopathy with histopathological features similar to IBM, and, less frequently, amyotrophic lateral sclerosis (16, 17). However, the distribution of weakness in MSP does not typically correspond to the canonical pattern in sporadic IBM, and no cognitive or behavioral features in this case suggest FTD. Myotonic dystrophy type 1 (DM1)—an autosomal dominant disorder caused by a trinucleotide repeat expansion in the dystrophia myotonica protein kinase gene—also warrants consideration in cases of adult-onset, gradually progressive neuromuscular weakness and cognitive dysfunction. Classic DM1 becomes symptomatic during the fourth decade of life at the latest, most commonly produces weakness in facial muscles, the sternocleidomastoid muscle, ankle dorsiflexors, and distal muscles of the forearms and hands, and is associated with cognitive changes that predominantly involve attention and executive dysfunction (18–20). These features and the lack of clinical or electrical myotonia make DM1 extremely unlikely in this case.
Question: What are therapeutic and prognostic considerations in this case?
AD and IBM are both gradually progressive diseases for which pharmacological treatments typically have modest benefits, at best. Consideration could be given to starting a cholinesterase inhibitor for cognitive impairment or deferring until the patient progresses to a stage of mild dementia when efficacy is more consistent (21). While effective in dermatomyositis and polymyositis, immunotherapies have not been found to have meaningful benefit for individuals with IBM (22). Nonpharmacological interventions for IBM are geared to optimize muscle strength and function in usual activities and reduce the risk of falling and aspiration, and these interventions include physical therapy, occupational therapy, speech therapy, exercise programs, and nutritional supports. Consultation with a pulmonologist or respiratory therapist can be useful to monitor the need for noninvasive ventilation, and consultation with a social worker can be useful to optimize caregiving support and resources (23).
AD and IBM are both characterized by phenotypic heterogeneity with respect to rates of symptom progression, as well as the specific profile of symptoms (3, 24, 25). For many patients, the most salient changes and greatest functional limitations during the mild to moderate stages of illness are related to progressive worsening of the more prominent symptoms at presentation. For this patient, progressive worsening would involve problems related to declining memory, dysphagia, and difficulties walking.
In the year following the initial presentation, our patient engaged with physical therapy, speech therapy, and otolaryngology services. He was hospitalized twice within a period of 3 months for aspiration pneumonia. He presented to the emergency department a third time when he tripped over a mat and hit the left side of his head on a desk, one of multiple falls in the interval since the evaluation. He continued to walk with a quad cane. His memory difficulties worsened such that he had difficulty placing recent significant life events in time and became more reliant on his wife to relay his history. He stopped driving, and his wife assumed responsibility for managing the family finances and overseeing his medications.
On neuropsychological reevaluation approximately 1 year after presentation, he was noted to be “bradyphrenic” and “tangential to a degree that he sometimes never answered the questions asked of him.” On testing, he exhibited “severe impairment in memory, language (semantic fluency), and executive functioning (working memory, set-shifting, processing speed)…(with) simple attention and visuoconstruction well below his baseline ability (but) areas of relative preservation.” He was started on donepezil, but this was discontinued shortly thereafter because of ongoing poor appetite and weight loss.
One and a half to 2 years after presentation, it was noted that he was very likely to sleep during the day when not actively engaged by others, despite sleeping 10–12 hours nightly using CPAP. He walked with a rollator walker outside of the home and independently within his apartment. He took 2–3 hours to finish a meal, adhering to a dysphagia diet supplemented with three to four cans of a high-calorie nutritional supplement daily. He was noted to be “not unhappy,” though without interest and motivation to engage in activities apart from watching television and listening to concerts at the assisted living residence where he and his wife had moved. He scored 11 out of 28 points on a modified version of the Mini-Mental State Examination (lower scores indicate greater impairment) that did not include drawing or writing tasks.
Notable changes following this visit included progressive daytime sleepiness, increased confusion in the evening, and transition to 24-hour care assistance. The patient died at age 77 from aspiration pneumonia, 12.5 years after he first experienced difficulties swallowing, 4.5 years following onset of memory difficulties, and approximately 2.5 years after presentation.
Neuropathology
The patient’s brain weight was 1,350 grams, which is in the range of normal (1,200–1,500 grams). Gross examination revealed mild atrophy of the parietal lobe. Serial transverse sections of the brainstem revealed loss of pigmentation in the substantia nigra. The locus coeruleus was not well visualized.
Microscopic sections revealed neuronal depletion of the locus coeruleus with tau-positive neurofibrillary tangles (NFTs) and threads in remaining neurons. Additionally, Lewy bodies, characterized by spherical eosinophilic cytoplasmic inclusions with a thin pale rim (Figure 2), were present and highlighted by alpha-synuclein. The neuronal population of the substantia nigra was depleted, and extraneuronal pigment was present, along with numerous Lewy bodies and scattered NFTs. The hippocampal formation exhibited moderate neuronal depletion involving pyramidal cell layer of CA1 to the subiculum, granulovacuolar degeneration, Hirano bodies, tau-positive NFTs and threads, and Lewy bodies. Tau-positive NFTs and threads and alpha-synuclein-positive Lewy bodies and neurites were likewise present in the amygdala and basal forebrain and diffusely throughout the cerebral cortex (less frequent in the occipital cortex). There were moderate to frequent extracellular beta-amyloid plaques involving the hippocampus, amygdala, basal forebrain, and cerebral cortex. Moderate to focally severe beta-amyloid deposition was observed in the walls of blood vessels in a widespread distribution throughout the cerebral cortex and leptomeninges, indicative of CAA.
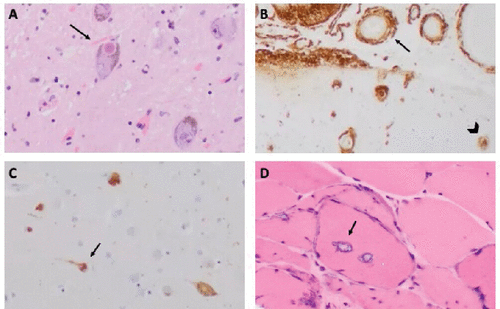
FIGURE 2. Histopathological findings in the brain and muscle of a 74-year-old patient with dysphagia, weakness, and memory lossa
aA: Microscopic view of the midbrain substantia nigra with hematoxylin and eosin (H&E) staining reveals cells with intracytoplasmic, round, eosinophilic inclusions diagnostic of Lewy bodies (arrow). B: Beta-amyloid immunohistochemistry of the hippocampus shows blood vessels with full thickness deposition of beta-amyloid within the walls of blood vessels (arrow) and extracellular plaques (arrowhead). C: Tau immunohistochemistry of the cingulate shows neurofibrillary tangles (arrow). D: A frozen section of the deltoid muscle with H&E staining shows vacuolar inclusions with basophilic rims (arrow) compatible with inclusion body myositis.
Deltoid muscle tissue was examined by using a standard protocol for muscle biopsies. Microscopic examination of the frozen section stained with hematoxylin and eosin revealed variability in myofiber size. Inflammatory infiltrates were not prominent. Some myofibers contained inclusions with a basophilic rim (“rimmed vacuoles”). A trichrome stain revealed increased endomysial connective tissue and highlighted rimmed vacuoles. Immunostaining revealed deposition of ubiquitin-binding protein p62 (p62), a marker of IBM, within affected muscle fibers (26, 27).
In summary, these findings culminated in neuropathological diagnoses of AD (Braak stage V of VI [28]), diffuse neocortical Lewy body disease (LBD), moderate to focally severe and widespread CAA, mild atherosclerosis, moderate arteriolar sclerosis, and IBM.
Discussion
These neuropathological findings raise several interesting questions, including whether there were clinical features suggesting LBD prior to death, how various factors may have contributed to the accelerated clinical course of our patient’s cognitive, motor, and functional decline, and whether common pathophysiological mechanisms might give rise to the simultaneous development of advanced AD, LBD, CAA, and IBM.
In retrospect, certain clinical features, such as jaw tremor, stooped posture, bradyphrenia, tangential thought process, and excessive daytime somnolence despite adequate sleep overnight and adherence with CPAP, represent clues to the possibility of LBD. The absence of cardinal features of parkinsonism (bradykinesia, rest tremor, and rigidity), formed visual hallucinations, dream enactment (by history and preserved atonia during rapid eye movement sleep on polysomnogram completed shortly prior to presentation), and marked fluctuations in cognition disqualifies a diagnosis of either probable or possible dementia with Lewy bodies (DLB) by consensus clinical diagnostic criteria (29). This dissociation between clinical symptoms and pathological findings illustrates the imperfect sensitivity of clinical diagnostic criteria for underlying neuropathology. Clinical and pathological studies of diffuse neocortical LBD pathology suggest a diagnostic sensitivity of approximately 75%–85% for the clinical diagnosis of probable DLB, which is reduced to 70% in cases with diffuse LBD and intermediate to high (Braak stages IV–VI) tau pathology (30, 31).
This patient’s progression from MCI to moderate dementia over the course of 2 years was atypically rapid for AD (24). His death from aspiration pneumonia 12–13 years after onset of neuromuscular weakness and 2–3 years following diagnosis of sporadic IBM was likewise atypical for IBM. IBM is associated with no effect on life expectancy among a majority of patients, although premature death due to aspiration pneumonia more than 15 years from the onset of symptoms and more than 10 years from diagnosis can occur among a subset of patients with more prominent early dysphagia (32, 33). The presence of comorbid AD, LBD, and arteriolosclerosis, which is common in this patient’s age range (34), likely accelerated his cognitive decline given evidence that LBD and cerebrovascular disease are independently associated with faster decline when comorbid with AD compared with AD occurring in isolation (35, 36). His history of traumatic brain injury (TBI) with cerebral contusion and subarachnoid hemorrhage also potentially contributed to rapid progression, with research suggesting faster progression of functional impairment among patients with AD and a history of TBI within 10 years of symptom onset, compared with patients with no history of TBI (37). Additionally, the combination of apathy related to neurodegenerative disease and dysphagia related to IBM could have contributed to the patient’s decline in cognitive and functional status, which aligns with evidence that nutritional factors influence rates of symptomatic progression and quality of life not only among individuals with dementia but also among those with myopathy and systemic inflammatory disorders (38–40).
An epidemiological association between AD, LBD, and IBM has not been established beyond rare case reports of patients with AD and IBM (41, 42). Citing similar histopathological features of AD, LBD, and IBM, including aggregates containing beta-amyloid, phosphorylated tau (p-tau), or alpha-synuclein in IBM muscle fibers, some investigators have proposed similar upstream disease mechanisms centered around the cytotoxicity of misfolded protein aggregates (43). However, in contrast to AD and LBD, IBM muscle fibers have been associated with the accumulation of dozens of additional aggregated molecules, and beta-amyloid, p-tau, and alpha-synuclein inclusions are present less consistently than p62, light chain 3, and transactive response DNA-binding protein of 43 kDa (TDP-43)—inclusions that reflect disruptions in the RNA metabolism response to stress and alterations in autophagy, the major intracellular degradation system by which old, damaged, or abnormal cytoplasmic materials are delivered to and degraded in the lysosome (3, 26, 27, 44). More importantly, immunological mechanisms appear to play a more consistent upstream role in IBM, evidenced histologically by the invasion of myofibers by cytotoxic T cells (a “signature” of highly differentiated CD8 T-cell effector memory and terminally differentiated effector cells), identification of anti-NT5C1A antibodies, strong genetic linkage of IBM to human leukocyte antigen gene variants associated with autoimmune disease, and microarray detection of abundant immune chemokine and cytokine transcripts in muscles affected by IBM (3, 45).
Mutations in genes including VCP and sequestosome 1 (the gene that encodes p62) cause MSP with histopathological features of myopathy resembling sporadic IBM but usually without inflammatory changes. This situation raises a question of whether a minority of cases of sporadic IBM without anti-NT5C1A antibodies and lymphocytic infiltrates could be driven predominantly by RNA stress granule dysfunction and impaired autophagy (16). These mechanisms have been implicated not only in frontotemporal lobar degeneration but also in AD and LBD (46–50). If neurodegeneration hinges on both cellular stress and inadequate cellular response mechanisms, then sporadic neurodegenerative diseases could arise in the context of grossly excessive stress, dysfunctional stress-response mechanisms, or some combination of the two. In AD, numerous types of cellular stress have been implicated, including beta-amyloid toxicity in the context of overproduction or impaired clearance (51), loss of presenilin function (52), cerebrovascular disease (53), impaired insulin signaling and other forms of metabolic stress (54), neuronal hyperexcitability (55), mitochondrial dysfunction (56), immunological dysregulation (57), infection (58), environmental toxins (59), and head trauma (60). AD has high comorbidity not only with LBD pathology but also with TDP-43 (61). It is plausible that in a case like the one discussed in this article, polygenic or unidentified genetic factors could confer vulnerability to multiple sources of age-related cellular stress to produce a “sporadic multisystem proteinopathy.” It is also plausible that our patient developed these multiple and substantial pathologies of the brain and muscle independently, for unrelated reasons.
1. : Memory dysfunction. Continuum 2018; 24:727–744Medline, Google Scholar
2. : The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2:a006171Crossref, Medline, Google Scholar
3. : Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 2019; 15:257–272Crossref, Medline, Google Scholar
4. : Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology 2014; 83:426–433Crossref, Medline, Google Scholar
5. : A symptoms and signs approach to the patient with neuromuscular weakness. Continuum 2022; 28:1580–1595Medline, Google Scholar
6. : Needle electromyography and histopathologic correlation in myopathies. Muscle Nerve 2019; 59:315–320Crossref, Medline, Google Scholar
7. : Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am 2002; 28:859–890Crossref, Medline, Google Scholar
8. : Immune-mediated conditions and antibodies associated with sporadic inclusion body myositis. Muscle Nerve 1998; 21:115–117Crossref, Medline, Google Scholar
9. : Cytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 2013; 73:408–418Crossref, Medline, Google Scholar
10. : Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann Neurol 2013; 73:397–407Crossref, Medline, Google Scholar
11. : Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153Crossref, Medline, Google Scholar
12. : The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 2022; 21:714–725Crossref, Medline, Google Scholar
13. : Cerebral microhemorrhages due to traumatic brain injury and their effects on the aging human brain. Neurobiol Aging 2018; 66:158–164Crossref, Medline, Google Scholar
14. : Cytoplasmic 5′-nucleotidase autoantibodies in inclusion body myositis: isotypes and diagnostic utility. Muscle Nerve 2014; 50:488–492Crossref, Medline, Google Scholar
15. : Disease specificity of autoantibodies to cytosolic 5′-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis 2016; 75:696–701Crossref, Medline, Google Scholar
16. : Multisystem proteinopathy: where myopathy and motor neuron disease converge. Muscle Nerve 2021; 63:442–454Crossref, Medline, Google Scholar
17. : Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 2001; 74:458–475Crossref, Medline, Google Scholar
18. : Brief assessment of cognitive function in myotonic dystrophy: multicenter longitudinal study using computer-assisted evaluation. Muscle Nerve 2022; 65:560–567Crossref, Medline, Google Scholar
19. : Cognitive impairment in adult myotonic dystrophies: a longitudinal study. Neurol Sci 2007; 28:9–15Crossref, Medline, Google Scholar
20. : Myotonic dystrophy: diagnosis, management and new therapies. Curr Opin Neurol 2014; 27:599–606Crossref, Medline, Google Scholar
21. : Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 2006:CD005593Medline, Google Scholar
22. : Clinical evaluation and management of inflammatory myopathies. Semin Neurol 2015; 35:347–359Crossref, Medline, Google Scholar
23. : Inclusion body myositis. Continuum 2022; 28:1663–1677Medline, Google Scholar
24. : Predicting progression of Alzheimer’s disease. Alzheimers Res Ther 2010; 2:2Crossref, Medline, Google Scholar
25. : The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol 2012; 8:451–464Crossref, Medline, Google Scholar
26. : Diagnostic value of markers of muscle degeneration in sporadic inclusion body myositis. Acta Myol 2011; 30:103–108Medline, Google Scholar
27. : Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol Commun 2013; 1:29Crossref, Medline, Google Scholar
28. : Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–259Crossref, Medline, Google Scholar
29. : Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89:88–100Crossref, Medline, Google Scholar
30. : Subtypes of dementia with Lewy bodies are associated with α-synuclein and tau distribution. Neurology 2020; 95:e155–e165Crossref, Medline, Google Scholar
31. : Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2018; 89:358–366Crossref, Medline, Google Scholar
32. : A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011; 134:3167–3175Crossref, Medline, Google Scholar
33. : Mortality and causes of death in patients with sporadic inclusion body myositis: survey study based on the clinical experience of specialists in Australia, Europe and the USA. J Neuromuscul Dis 2016; 3:67–75Crossref, Medline, Google Scholar
34. : Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis 2021; 79:389–400Crossref, Medline, Google Scholar
35. : Trajectories of cognitive decline in brain donors with autopsy-confirmed Alzheimer disease and cerebrovascular disease. Neurology 2022; 98:e2454–e2464Crossref, Medline, Google Scholar
36. : Clinical trajectories at the end of life in autopsy-confirmed dementia patients with Alzheimer disease and Lewy bodies pathologies. Neurology 2022; 98:e2140–e2149Crossref, Medline, Google Scholar
37. : The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer’s disease: the Cache County Dementia Progression Study. Int Psychogeriatr 2014; 26:1593–1601Crossref, Medline, Google Scholar
38. : Nutritional status and body composition in Korean myopathy patients. Clin Nutr Res 2016; 5:43–54Crossref, Medline, Google Scholar
39. : Nutrition and connective tissue disease. Clin Dermatol 2022; 40:166–172Crossref, Medline, Google Scholar
40. : Nutritional status is associated with faster cognitive decline and worse functional impairment in the progression of dementia: the Cache County Dementia Progression Study. J Alzheimers Dis 2016; 52:33–42Crossref, Medline, Google Scholar
41. : Inclusion-body myositis associated with Alzheimer’s disease. Case Rep Med 2013; 2013:536231Crossref, Medline, Google Scholar
42. : Inclusion body myositis in Alzheimer’s disease. Acta Neurol Scand 2011; 124:215–217Crossref, Medline, Google Scholar
43. : Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 2008; 116:583–595Crossref, Medline, Google Scholar
44. : Autophagy: renovation of cells and tissues. Cell 2011; 147:728–741Crossref, Medline, Google Scholar
45. : Highly differentiated cytotoxic T cells in inclusion body myositis. Brain 2019; 142:2590–2604Crossref, Medline, Google Scholar
46. : Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One 2010; 5:e9313Crossref, Medline, Google Scholar
47. : Autophagy mediators (FOXO1, SESN3 and TSC2) in Lewy body disease and aging. Neurosci Lett 2018; 684:35–41Crossref, Medline, Google Scholar
48. : Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc Natl Acad Sci USA 2022; 119:e2211999119Crossref, Medline, Google Scholar
49. : Stress granules and neurodegeneration. Nat Rev Neurosci 2019; 20:649–666Crossref, Medline, Google Scholar
50. : Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 2010; 133:1352–1367Crossref, Medline, Google Scholar
51. : The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016; 8:595–608Crossref, Medline, Google Scholar
52. : The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA 2007; 104:403–409Crossref, Medline, Google Scholar
53. : Cerebral small vessel disease and Alzheimer’s disease: a review. Front Neurol 2020; 11:927Crossref, Medline, Google Scholar
54. : Impaired insulin signalling and allostatic load in Alzheimer disease. Nat Rev Neurosci 2022; 23:215–230Crossref, Medline, Google Scholar
55. : Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology 2020; 95:e2259–e2270Crossref, Medline, Google Scholar
56. : The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement 2023; 19:333–342Crossref, Medline, Google Scholar
57. : Peripheral and central immune system crosstalk in Alzheimer disease: a research prospectus. Nat Rev Neurol 2021; 17:689–701Crossref, Medline, Google Scholar
58. : Do infections have a role in the pathogenesis of Alzheimer disease? Nat Rev Neurol 2020; 16:193–197Crossref, Medline, Google Scholar
59. : Environmental toxins and Alzheimer’s disease progression. Neurochem Int 2020; 141:104852Crossref, Medline, Google Scholar
60. : Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 2022; 91:498–507Crossref, Medline, Google Scholar
61. : TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain 2016; 139:2983–2993Crossref, Medline, Google Scholar



