Primary CNS Lymphoma and Secondary Causes of Mania: A Case Report and Literature Review
Mania can be from primary etiology, as seen in bipolar disorder, or resulting from secondary causes. In DSM-5, secondary bipolar disorder is characterized as disturbances generally caused by another medical condition or induced by a substance or medication. These disturbances cause the same symptoms as primary mania, including a prominent and persistent period of abnormally elevated, expansive, or irritable mood and an abnormally increased activity or energy. In addition, secondary mania must be supported by evidence from the history, physical examination, or laboratory findings to suggest the direct pathophysiological consequence of another medical condition or exposure to a medication (1). Common medical causes include neurological conditions, such as tumors, frontotemporal dementia, stroke, and traumatic brain injury (TBI), whereas medications and substance causes can include corticosteroids and stimulants (2). Secondary mania often develops within a short period (hours to days) after the physiological insult and may occur throughout the lifespan. The prevalence of secondary mania is variable due to its multitude of potentiating factors. In a retrospective chart review of 755 patients evaluated by the psychiatry consultation service in a general hospital, it was found that in a 1-year period, 13 of 15 (87%) patients diagnosed with mania met criteria for secondary mania (3). Here, we discuss a case of a man in his late 60s who developed mania after diagnosis and treatment of his primary central nervous system lymphoma (PCNSL), in which the cause of the mania was likely multifactorial.
CASE REPORT
A man in his late 60s, with no prior psychiatric or substance use diagnoses or hospitalizations, was brought to psychiatric attention following chemotherapy for treatment of PCNSL. Pertinent past medical history included coronary artery disease managed with aspirin.
The patient’s initial presentation to his primary care physician in March occurred after having a decrease in mood as a result of significant physical limitations after falling off a ladder. Over the course of the next 6 weeks, his mental status further deteriorated. He endorsed symptoms of “brain fog” characterized by difficulty with cognitive functioning and higher-level processing. He felt increasingly fatigued and tired and was sleeping up to 22 hours a day. His wife described that his presentation was different from his baseline (normally outgoing and expressive). Blood work demonstrated the patient’s complete blood count (CBC), comprehensive metabolic panel (CMP), thyroid-stimulating hormone, vitamin B12 and thiamin levels, erythrocyte sedimentation rate, and C-reactive protein inflammatory markers to be within normal limits, as well as negative hepatitis B, hepatitis C, and HIV results. Head imaging in May (computerized tomography [CT] and MRI) revealed two intracranial masses involving the right basal ganglia and left frontal lobe with mild vasogenic edema and mass effect (Figure 1). The patient was referred to a nearby tertiary care hospital, where a stereotactic CT-guided biopsy indicated an aggressive, myeloid differentiation primary response 88 (MYD88) positive, B-cell lymphoma. Further imaging on positron emission tomography CT (PET-CT) showed PCNSL without extracranial involvement. Bone marrow biopsy was negative for lymphoma.
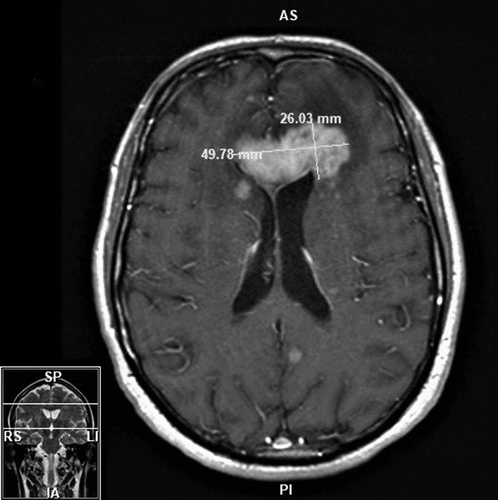
FIGURE 1. Axial T1 MRI of a mass extending along the genu of the corpus callosum with multiple smaller enhancing lesions in both cerebral hemispheres without infarction or hemorrhage in a patient who sustained secondary mania in the setting of primary central nervous system lymphoma and its subsequent treatmenta
a The mass measurement was 49.78 x 26.03 mm. AS=anterior superior; IA=inferior anterior; LI=left inferior; PI=posterior inferior; RS=right superior; SP=superior posterior.
The patient was started on high-dose methotrexate (HDM; 8.3–10.8 g), rituximab, and temozolomide (MRT) induction therapy. He completed a total of nine cycles over 4 months starting in June. Regular blood monitoring revealed persistently high serum concentration of methotrexate (>0.10 mcmol/L). Over this time, he received 8-mg intravenous dexamethasone every other week (for a total of 10 doses) for vasogenic edema secondary to tumor mass effect. In addition, five scattered doses of baclofen (10 mg) were given for hiccups. A surveillance brain MRI after the first two cycles of MRT showed resolution of enhancement and decrease in multiple areas of the abnormal T2 signal. Follow-up imaging in August noted regression of the tumor size, as well as an incidental, asymptomatic right-sided 7-mm chronic subdural hematoma (SDH) with no noted mass effect or midline shift (Figure 2).
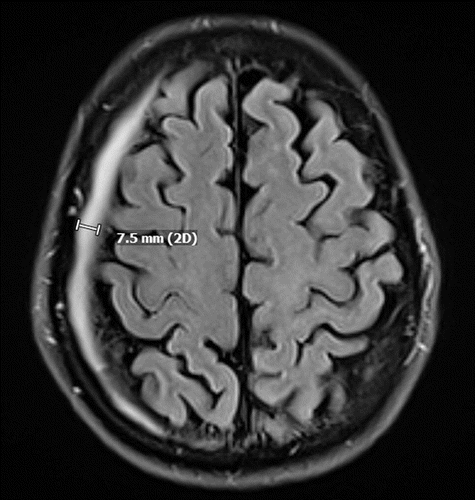
FIGURE 2. Axial computerized tomography demonstrating a right-sided 7-mm chronic subdural hematoma in a patient who sustained secondary mania in the setting of primary central nervous system lymphoma and its subsequent treatmenta
a 2D=two-dimensional.
In September, a repeat MRI showed resolution of the patient’s PCNSL, and he was determined to have achieved complete remission. A repeat PET-CT for tumor restaging—part of the process for his evaluation prior to bone marrow transplant—was negative for extracranial PCNSL. However, there was an interval increase in the size of the SDH on brain MRI, which resulted in temporary postponement of the patient’s stem cell transplant (SCT) so that the hematoma could resolve. Findings from laboratory studies conducted as part of his pre-SCT evaluation (coagulation studies, vitamin D, iron panel, and urinalysis) were all within normal limits. His infectious disease panel was nonreactive for cytomegalovirus, hepatitis B core antibodies, hepatitis B surface antigen, hepatitis B virus nucleic acid testing, hepatitis B virus antibodies, hepatitis C virus nucleic acid testing, HIV-1/-2, HIV-1 nucleic acid testing, human T-lymphotropic virus -I/-II, syphilis antibodies, Trypanosoma cruzi antibody, and West Nile virus nucleic acid testing. Chromosome analysis showed normal karyotype of 46, XY.
In early October, the patient’s wife expressed concern that he was exhibiting impulsive behavior, disinhibition, irritability, and mood changes. A repeat head CT showed stable but slightly increased size of the SDH, with minimally associated mass effect. Blood work was noncontributory for CBC, CMP, lipids, and thyroid panel. Over the course of the month, the patient’s condition progressively worsened, and he developed new daily symptoms of inflated self-esteem, irritability, lability, and elevated mood. According to his wife, he also had a decreased need for sleep and demonstrated pressured speech. In addition, he became easily distracted and disorganized to the extent that he was unable to complete his usual tasks as a teacher. He was also noted to have increased goal-directed activities, such as hypersexuality and hyperreligiosity, as well as irresponsible behaviors, such as nondiscretionary spending.
Despite these documented behavioral changes, no focal neurological deficits or organ-specific symptoms were found. He was evaluated by neurology and neurosurgery teams, who described him as “disorganized, grandiose, and loquacious” (quoted from the patient’s chart). He was noted to be careless when performing the clock-drawing portion of the cognitive examination. His changes in behavior raised concerns for PCNSL recurrence, with frontal lobe involvement. A repeat brain MRI showed a slight decrease in the size of the SDH, with no indication of PCNSL recurrence. Cerebrospinal fluid analysis revealed no concerns regarding an infectious or autoimmune etiology to his presentation.
The patient was referred for a psychiatric evaluation in late October, where it was concluded that he was having a manic episode. His Young Mania Rating Scale (YMRS) score was 31/60, and his Patient Health Questionnaire–9 score was 0/27. He was subsequently admitted to a psychiatric hospital. Over the course of 10 days, the patient was stabilized on valproic acid (500 mg b.i.d.) and olanzapine (5 mg qAM and 10 mg q.h.s.). His YMRS score on discharge was 15, with sufficient functional improvement of his manic symptoms to warrant an outpatient level of care. Just under 3 weeks after hospital discharge (late November), his YMRS score was 0; in mid-December, he was able to successfully undergo an autologous SCT. Over the next 3–4 months, he underwent a slow and gradual titration off his psychotropic medications with no recurrence of his manic symptoms.
DISCUSSION
This case provides an opportunity for a valuable discussion regarding secondary mania, particularly the potential underlying etiologies pertinent to our patient. To begin, we identify secondary mania as the most appropriate diagnosis considering the late age at onset (new-onset mania is rare after age 50) and the lack of personal or family history of bipolar disorder (4, 5). Additionally, multiple factors in our patient’s case could have acted as a catalyst or exacerbating component to his manic episode. Here, we will highlight each of these factors and discuss the literature surrounding their potential to influence a manic presentation. Lastly, we will provide future directions on how such a complex, multifactorial case of secondary mania can be optimally managed in the future.
Medical Conditions
Lymphoma.
Our patient’s brain cancer could have been a source of his symptoms, because a tumor’s location and size can cause disruptions in neurocircuitry that could result in psychiatric consequences (6). Brain tumors have been correlated with depression, demoralization, anxiety, posttraumatic stress disorder, and mania (7). More specifically, PCNSL has been shown to be highly correlated with psychiatric symptoms at presentation. In fact, a review of 248 patients with PCNSL found that 43% initially presented with neuropsychiatric symptoms that eventually led to the discovery of the underlying lymphoma (8). More recently, in a study published in 2020, Sharma et al. (9) noted that 9% of 232 patients presented primarily with neuropsychiatric symptoms leading to their PCNSL diagnosis. These included symptoms commonly seen in mania, such as agitation (30%), anxiety (30%), hallucinations (25%), delusions (20%), paranoia (10%), and disinhibition (10%). In addition, a case report published in 2018 described a patient whose PCNSL relapse presented solely as new-onset psychosis, characterized by irritability, disinhibition, and disorganized behavior, requiring 6 months of antipsychotic treatment (10). Our patient’s presentation reflects attributes similar to those described in the literature.
Tumor location.
Our patient’s tumor was located along the genu of the corpus callosum, involving the right more than the left frontal lobe. Frontal lobe tumors have been shown to cause disinhibition, impulsivity, mood lability, reduced executive functioning, and poor judgment (2, 9). Furthermore, studies have suggested an association with right-sided lesions in patients with brain injury who developed secondary mania (11, 12). In a 2020 systematic review of 201 cases of adult-onset mania following a focal brain lesion, most patients had lesions involving the right hemisphere (60.7%), preferentially located in the frontal lobe (13). Our patient’s tumor location could have disturbed interhemispheric connectivity, which may have contributed to his manic presentation.
TBI and subdural hematoma.
Our patient’s treatment course was also complicated by an SDH, measuring 7.5 mm in its greatest thickness, along the right cerebral convexity. Traumatic SDH often occurs from a fall (14). Although our patient did not definitively recall falling, it is not unforeseen, given that 80% of patients have little to no memory of the inciting event (14). TBI can cause changes in one’s behavior, such as affective lability and irritability (15). More specifically, associated symptoms of anger, loss of temper, impatience, and annoyance resulting from TBI all appear similar to symptoms of mania and can be triggered by even mild stressors (16).
Medications
Corticosteroids.
In cancer patients, corticosteroids are often a common cause of mania (17). Patients exposed to steroids have a greater than fourfold increased rate of developing mania compared with nonexposed patients (18). Symptoms of corticosteroid-induced mania include mood lability, euphoria, irritability, anxiety, insomnia, and psychosis (3, 17). The most significant risk factor for developing neuropsychiatric effects from steroids is administration of a higher daily dosage (defined as >40 mg daily prednisone equivalence) (19). If high-dose corticosteroids are planned as part of a chemotherapy regimen and mania is a concern, subsequent cycles may be preceded by initiation of valproate or lithium (20). For 90% of patients, the onset of psychiatric symptoms is within the first 6 weeks of initiating or increasing corticosteroid treatment (21). In our case, the patient received intravenous dexamethasone (prednisone equivalence of 50 mg) every other week for a total of 10 doses over the 4 months prior to his mania.
Methotrexate.
HDM (>500 mg/m2) is commonly used in combination with rituximab and temozolomide for induction therapy in the treatment of PCNSL (20). Our patient was given HDM with elevated serum levels throughout most of his 4 months of chemotherapy. Although rare, potential neuropsychiatric side effects of HDM are related to both dose and duration of drug exposure (22, 23). Cognitive and psychiatric disturbances, including mania, have been reported with HDM and have resolved with methotrexate discontinuation (24, 25).
Baclofen.
To date, the literature includes three case reports, to our knowledge, of baclofen-induced mania in patients without previous mood disorders (with doses ranging from 60 to 180 mg/day) (26–28). The mood-elevating properties of baclofen are thought to be dose dependent (29). The literature estimates that baclofen can induce manic or hypomanic symptoms in up to 15% of patients (30). Because our patient’s dose and frequency of baclofen were low, it is unlikely that this medication played a prominent role in his mania; however, for the purposes of a comprehensive discussion, baclofen was included in this review.
Management and Future Directions
Ensuring an appropriate diagnostic evaluation of a patient with manic symptoms is crucial. As noted in the introduction, multiple factors from the patient’s history can delineate between primary and secondary mania (1). It is essential to conduct an appropriate evaluation of the potential structural, metabolic, infectious, neoplastic, autoimmune, and medication etiologies if secondary mania is suspected (9). Considering the heterogeneous presentation, treatment of both the mania and the underlying medical conditions is key (31, 32). Secondary mania is often managed in an inpatient setting with mood stabilizers and antipsychotics, with close consideration for potential hepatic or renal impairment and cytochrome P450 interactions (2, 33). Electroconvulsive therapy should be considered in patients whose mania is unresponsive to pharmacological interventions (34).
Depending on the underlying psychiatric comorbidities, the time frame for weaning off antimanic medications and coordination of longitudinal care may differ (31). Upon symptom stabilization, the speed of the potential medication tapering depends on symptom recurrence and intolerance to medication withdrawal (35). If secondary mania occurs in the context of no clear underlying or additional psychiatric diagnoses, a slow wean off the antimanic medications would be appropriate over a few months. If this wean is well tolerated, there may not be a need for long-term psychiatric follow-up. If secondary mania occurs in the context of comorbid major depression, there may be more caution with weaning off antimanic medications (particularly if a concomitant antidepressant is actively being prescribed due to the risk of mania or hypomania induction) (36). These patients would benefit from longitudinal psychiatric follow-up. If there is an underlying bipolar disorder, the antimanic medications will likely be continued longitudinally with close psychiatric follow-up to ensure necessary dosing adjustment if breakthrough occurs (32, 35, 37).
CONCLUSIONS
The potential causes of mania arising from the diagnosis, treatment, and sequelae of PCNSL are complex and multifactorial. Ensuring a comprehensive evaluation of the underlying causes and exacerbating factors is essential to ensure timely and appropriate treatment.
1
2 : The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry, 3rd ed. Washington, DC, American Psychiatric Association Publishing, 2019Google Scholar
3 : Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci 1989; 1:398–400Link, Google Scholar
4 : Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord 2009; 116:176–183Crossref, Medline, Google Scholar
5 : Mania in the medically ill. Curr Psychiatry Rep 2007; 9:232–235Crossref, Medline, Google Scholar
6 : Interhemispheric functional disconnection because of abnormal corpus callosum integrity in bipolar disorder type II. BJPsych Open 2016; 2:335–340Crossref, Medline, Google Scholar
7 : Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011; 12:160–174Crossref, Medline, Google Scholar
8 : Primary central nervous system lymphoma: presentation, diagnosis and staging. Neurosurg Focus 2006; 21:E15Crossref, Medline, Google Scholar
9 : Psychiatric disturbance or Parkinsonism as a presentation of CNS lymphoma: observational retrospective study and review of literature. Am J Clin Oncol 2020; 43:727–733Crossref, Medline, Google Scholar
10 : Psychosis as an indicator of recurrent non-Hodgkin’s lymphoma: a rare presentation. Gen Psychiatr 2018; 31:e000005Medline, Google Scholar
11 : Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry 1988; 145:172–178Crossref, Medline, Google Scholar
12 : Mechanisms of mania after brain injury: 12 case reports and review of the literature. J Nerv Ment Dis 1988; 176:87–100Crossref, Medline, Google Scholar
13 : Right-sided brain lesions predominate among patients with lesional mania: evidence from a systematic review and pooled lesion analysis. Transl Psychiatry 2020; 10:139Crossref, Medline, Google Scholar
14 : Subdural hematoma presenting as psychogenic nausea. J Psychiatr Pract 2021; 27(5):395–399Google Scholar
15 : Mood disorders after TBI. Psychiatr Clin North Am 2014; 37:13–29Crossref, Medline, Google Scholar
16 :
17 : Clinical Manual of Psychosomatic Medicine: A Guide to Consultation-Liaison Psychiatry, 2nd ed. Washington, DC, American Psychiatric Publishing, 2012Google Scholar
18 : Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry 2012; 169:491–497Crossref, Medline, Google Scholar
19 : Mood and cognitive changes during systemic corticosteroid therapy. Prim Care Companion J Clin Psychiatry 2001; 3:17–21Crossref, Medline, Google Scholar
20 : Adjuvant therapy of melanoma with interferon-alpha-2b is associated with mania and bipolar syndromes. Cancer 2000; 89:356–362Crossref, Medline, Google Scholar
21 Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther 1972; 13:694–698Crossref, Medline, Google Scholar
22 : Toxicity of high-dose methotrexate administration at the Sidney Kimmel Cancer Center (SKCC): a retrospective review to guide establishment of an outpatient treatment program. J Clin Oncol 2020; 38:30Crossref, Google Scholar
23 : Therapeutic use and toxicity of high-dose methotrexate. UpToDate. https://www.uptodate.com/contents/therapeutic-use-and-toxicity-of-high-dose-methotrexateGoogle Scholar
24 : Neuropsychologic effects of chemotherapy on children with cancer: a longitudinal study. J Clin Oncol 1996; 14:2826–2835Crossref, Medline, Google Scholar
25 : Contribution of methotrexate in precipitation of manic episode in bipolar affective disorder explored: a case report. Ther Adv Psychopharmacol 2013; 3:251–254Crossref, Medline, Google Scholar
26 : Behavioral disinhibition with baclofen. J Clin Psychopharmacol 2010; 30:759–760Crossref, Medline, Google Scholar
27 : A case of mania associated with high-dose baclofen therapy. J Clin Psychopharmacol 1992; 12:215–217Medline, Google Scholar
28 : Baclofen-induced manic symptoms: case report and systematic review. Psychosomatics 2014; 55:326–332Crossref, Medline, Google Scholar
29 : Baclofen abuse due to its hypomanic effect in patients with alcohol dependence and comorbid major depressive disorder. Clin Psychopharmacol Neurosci 2017; 15:187–189Crossref, Medline, Google Scholar
30 : A review of the potential mechanisms of action of baclofen in alcohol use disorder. Front Psychiatry 2018; 9:506Crossref, Medline, Google Scholar
31 : Mania secondary to focal brain lesions: implications for understanding the functional neuroanatomy of bipolar disorder. Bipolar Disord 2016; 18:205–220Crossref, Medline, Google Scholar
32 : Bipolar disorder in older adults: a critical review. Bipolar Disord 2004; 6:343–367Crossref, Medline, Google Scholar
33 : Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database Syst Rev 2013; 5:CD006932Google Scholar
34 : Electroconvulsive therapy in geriatric psychiatry: a selective review. Psychiatr Clin North Am 2018; 41:79–93Crossref, Medline, Google Scholar
35 : Geriatric bipolar disorder: maintenance treatment. UpToDate. https://www.uptodate.com/contents/geriatric-bipolar-disorder-maintenance-treatment/printGoogle Scholar
36 : The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry 2010; 71:1488–1501Crossref, Medline, Google Scholar
37 : The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry 2003; 160:2099–2107Crossref, Medline, Google Scholar



