Interactions Between Neuropsychiatric Symptoms and Alzheimer's Disease Neuroimaging Biomarkers in Predicting Longitudinal Cognitive Decline
Abstract
Objective
To examine interactions between Neuropsychiatric symptoms (NPS) with Pittsburgh Compound B (PiB) and fluorodeoxyglucose positron emission tomography (FDG‐PET) in predicting cognitive trajectories.
Methods
We conducted a longitudinal study in the setting of the population‐based Mayo Clinic Study of Aging in Olmsted County, MN, involving 1581 cognitively unimpaired (CU) persons aged ≥50 years (median age 71.83 years, 54.0% males, 27.5% APOE ɛ4 carriers). NPS at baseline were assessed using the Neuropsychiatric Inventory Questionnaire (NPI‐Q). Brain glucose hypometabolism was defined as a SUVR ≤ 1.47 (measured by FDG‐PET) in regions typically affected in Alzheimer's disease. Abnormal cortical amyloid deposition was measured using PiB‐PET (SUVR ≥ 1.48). Neuropsychological testing was done approximately every 15 months, and we calculated global and domain‐specific (memory, language, attention, and visuospatial skills) cognitive z‐scores. We ran linear mixed‐effect models to examine the associations and interactions between NPS at baseline and z‐scored PiB‐ and FDG‐PET SUVRs in predicting cognitive z‐scores adjusted for age, sex, education, and previous cognitive testing.
Results
Individuals at the average PiB and without NPS at baseline declined over time on cognitive z‐scores. Those with increased PiB at baseline declined faster (two‐way interaction), and those with increased PiB and NPS declined even faster (three‐way interaction). We observed interactions between time, increased PiB and anxiety or irritability indicating accelerated decline on global z‐scores, and between time, increased PiB and several NPS (e.g., agitation) showing faster domain‐specific decline, especially on the attention domain.
Conclusions
NPS and increased brain amyloid deposition synergistically interact in accelerating global and domain‐specific cognitive decline among CU persons at baseline.
Highlights
In cognitively unimpaired older adults.
Neuropsychiatric symptoms and brain amyloid synergistic interaction.
Lead to accelerated cognitive decline.
Neuropsychiatric symptoms (NPS) are very common in Alzheimer's Disease (AD). Some estimate that up to 97% of AD patients develop NPS at some point in the course of their illness (1). We and others (2, 3) have demonstrated that the prevalence of NPS ranges between 25% in cognitively unimpaired (CU) persons to approximately 50% in persons with mild cognitive impairment (MCI), with the most frequent NPS being depression, irritability, and apathy (3). As expected, the frequency of NPS is even higher in persons with AD with up to 80% exhibiting at least one NPS (2).
We and others have reported that NPS are associated with an increased risk of new onset of MCI (4, 5, 6) or dementia (7, 8, 9, 10, 11) as well as decline in cognitive trajectories (12, 13). NPS in the context of AD spectrum lead to accelerated cognitive and functional decline, profound caregiver distress, early institutionalization, and increased mortality. However, despite the enormous impact of NPS on patients, caregivers, and society at large, the mechanism linking AD biomarkers to NPS and cognitive decline over a longitudinal follow up remains unclear.
Biomarkers can identify persons on the AD spectrum early in the course of the disease and before the onset of clinical signs (14). Beta‐amyloid (Aβ) deposition can be visualized in vivo by amyloid brain imaging using various types of tracers (15). Amyloid imaging is a biomarker of AD, and brain glucose hypometabolism as measured by fluorodeoxyglucose positron emission tomography (FDG‐PET) is a biomarker of neurodegeneration (14).
While we and others have examined anxiety, depression and their interaction with neuroimaging biomarkers in predicting MCI (16) and cognitive decline (17), studies involving a broad spectrum of NPS are lacking.
Therefore, we sought to examine interactions between NPS as measured by the Neuropsychiatric Inventory Questionnaire (NPI‐Q) and neuroimaging biomarkers, that is, amyloid imaging and FDG‐PET in predicting global and domain‐specific cognitive decline in community‐dwelling older adults. The primary question was if any NPS, as measured by NPI‐Q, interacted with amyloid deposition or glucose hypometabolism in predicting cognitive decline. In addition, we examined specific NPS.
We hypothesized that there would be an interaction between NPS and neuroimaging biomarkers in increasing the rate of cognitive decline in community‐dwelling individuals.
METHODS
Study Design and Sample
We conducted a prospective cohort study in the setting of the population‐based Mayo Clinic Study of Aging (MCSA) in Olmsted County, MN, USA. Details of the study procedures have been reported elsewhere (18).
We included 1581 CU participants ≥50 years who underwent baseline NPS assessment and amyloid‐PET and FDG‐PET neuroimaging, with the majority having repeated cognitive testing after approximately every 15 months.
Participants were followed forward in time for a median of 6.2 years to examine interactions between baseline NPS and amyloid‐PET as well as FDG‐PET with longitudinal changes in global and domain specific (memory, attention, language, visuospatial) cognitive z‐scores.
The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards, and informed consent for participation was obtained from every participant.
Cognitive Evaluation
MCSA participants underwent face‐to‐face evaluations including risk factor ascertainment (including NPI‐Q) and baseline evaluation (including Clinical Dementia Rating Scale) (19) performed by a nurse or study coordinator; a neurologic evaluation including a neurologic interview, Short Test of Mental Status (20), and neurologic examination performed by behavioral neurologists; and neuropsychological evaluation of four cognitive domains: memory (delayed recall trials from the Auditory Verbal Learning Test (21) and the Wechsler Memory Scale–Revised (22), Logical Memory and Visual Reproduction subtests); language (Boston Naming Test (23) and category fluency); visuospatial (Wechsler Adult Intelligence Scale–Revised (23), Picture Completion and Block Design subtests); and executive function (Trail Making Test Part B (24) and the Wechsler Adult Intelligence Scale–Revised (25), Digit Symbol subtest). All tests were administered by psychometrists and supervised by neuropsychologists. An expert consensus panel of physicians, neuropsychologists, and nurses or study coordinators reviewed the data and determined if a participant was CU, had MCI (based on the revised Mayo Clinic criteria (26) or dementia. In this analysis we included only individuals who were CU; participants with MCI or dementia were excluded for the current analysis at baseline. Classification of CU was based on normative data developed in this community (27, 28, 29, 30).
We further created domain‐specific cognitive z‐scores by z‐scoring the averages of the test‐specific z‐scores, and additionally created a global z‐score by z‐scoring the averages of the domain‐specific z‐scores. The outcome of interest for the linear mixed‐effect model analyses was the longitudinal change in global and domain‐specific (i.e., memory, attention/executive function, language, visuospatial skills) cognitive z‐scores.
Measurement of Neuropsychiatric Symptoms
NPS were measured by using the NPI‐Q (31) which was administered as a structured interview to an informant, usually the spouse. The NPI‐Q is a shorter version of the Neuropsychiatric Inventory (NPI) and is a clinical instrument that is cross‐validated with the standard NPI (31). We considered the NPI‐Q an appropriate screening instrument because it assesses a broad variety of neuropsychiatric symptoms and was also selected by the Uniform Data Set Initiative of the National Institute on Aging (32). The NPI‐Q is designed to obtain information on 12 behaviors (i.e., agitation, delusion, hallucination, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep, and eating/appetite). A severity scale has scores ranging from 1 to three points (1 = mild; 2 = moderate; and 3 = severe) and a scale for assessing caregiver distress has scores ranging from 0 to five points (0 = no distress; 1 = minimal distress; 2 = mild distress; 3 = moderate distress; 4 = severe distress; and 5 = extreme distress).
PiB‐PET Acquisition
Amyloid PET imaging was performed using the Pittsburgh Compound B (PiB) tracer. Details on PiB‐PET imaging in the MCSA have been published elsewhere (33, 34). Briefly, PiB scans, consisting of four 5‐min dynamic frames, were acquired 40–60 min after intravenous injection with 292–728 MBq of 11C‐PiB. We used an in‐house, fully automated image processing pipeline to analyze images. Herein, image voxel values were extracted from automatically labeled regions of interest (ROI) propagated from regions defined on each participant's own magnetic resonance imaging (MRI). The prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROI were normalized to the cerebellar gray matter to form a global amyloid PET standardized uptake value ratio (SUVR). We defined abnormal PiB‐PET retention (PiB‐PET+) by an SUVR ≥1.48, which is the current cut‐off used in the MCSA (33, 35). We ran the linear‐mixed effects models with continuous, z‐scored PiB‐PET SUVR.
FDG‐PET Acquisition
FDG‐PET imaging which consisted of four 2‐min dynamic frames, was performed 30 min after injecting 366–399 MBq of 18fluorodeoxyglucose intravenously. Images were analyzed using our in‐house fully automated image processing pipeline (36) in which image voxel values were extracted from automatically labeled cortical ROI (37) After combining the left and right regions from the Atlas, there were 19 ROI and the meta‐region of interest consisted of bilateral angular gyrus, posterior cingulate/precuneus, and inferior temporal cortical regions from both hemispheres and was identified as AD signature ROI (38, 39). SUVR was formed by the ratio of this AD signature ROI and two reference regions, namely the pons and the cerebellar vermis which have preserved glucose metabolism in AD (40). Participants were classified as having glucose hypometabolism, which is a measure of neurodegeneration as defined by NIA‐AA criteria (N+) (14) based on SUVR of ≤1.47 (33). We ran the linear‐mixed effects models with continuous, z‐scored FDG‐PET SUVR. We additionally flipped the sign of the z‐score so that higher values would correspond with a worsening of the biomarker, thereby allowing for a similar interpretation as for the PiB‐PET analysis.
Statistical Analysis
We conducted linear mixed‐effect models with random participant‐specific intercepts and slopes over time to examine the associations and interactions between baseline NPS with brain amyloid deposition (as measured by PiB‐PET) or glucose hypometabolism (as measured by FDG‐PET) in predicting longitudinal change in global and domain‐specific (i.e. attention/executive function, memory, visuospatial, language) cognitive z‐scores over time. We ran the models with continuous, z‐scored PiB‐PET as well as FDG‐PET SUVR (with sign reversed for interpretation purposes for the FDG‐PET SUVR). All models included NPS at baseline, PET imaging at baseline, time in years from baseline and their interactions. All models were adjusted for age at baseline, sex, education, and previous cognitive testing experience (Yes/No). We conducted this analysis separately for the 12 NPS as assessed by the NPI‐Q, and for presence of any NPS as well as NPS severity. For each model, we computed beta coefficients, 95% confidence intervals (CIs), and p‐values.
For visual display of data, we plotted the linear mixed effects model for PiB‐PET SUVR (average vs. 1 standard deviation (SD) above the mean) and presence of any NPS (Yes/No) predicting the attention z‐score, as well as presence of anxiety predicting the global cognition z‐score to show the trajectories over time for individuals in these groups (Figures 1, 2, 3). Statistical testing was performed at the conventional two‐tailed alpha level of 0.05. All analyses were performed using SAS System, version 9.4 software (SAS Institute, Cary, NC) and R, version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
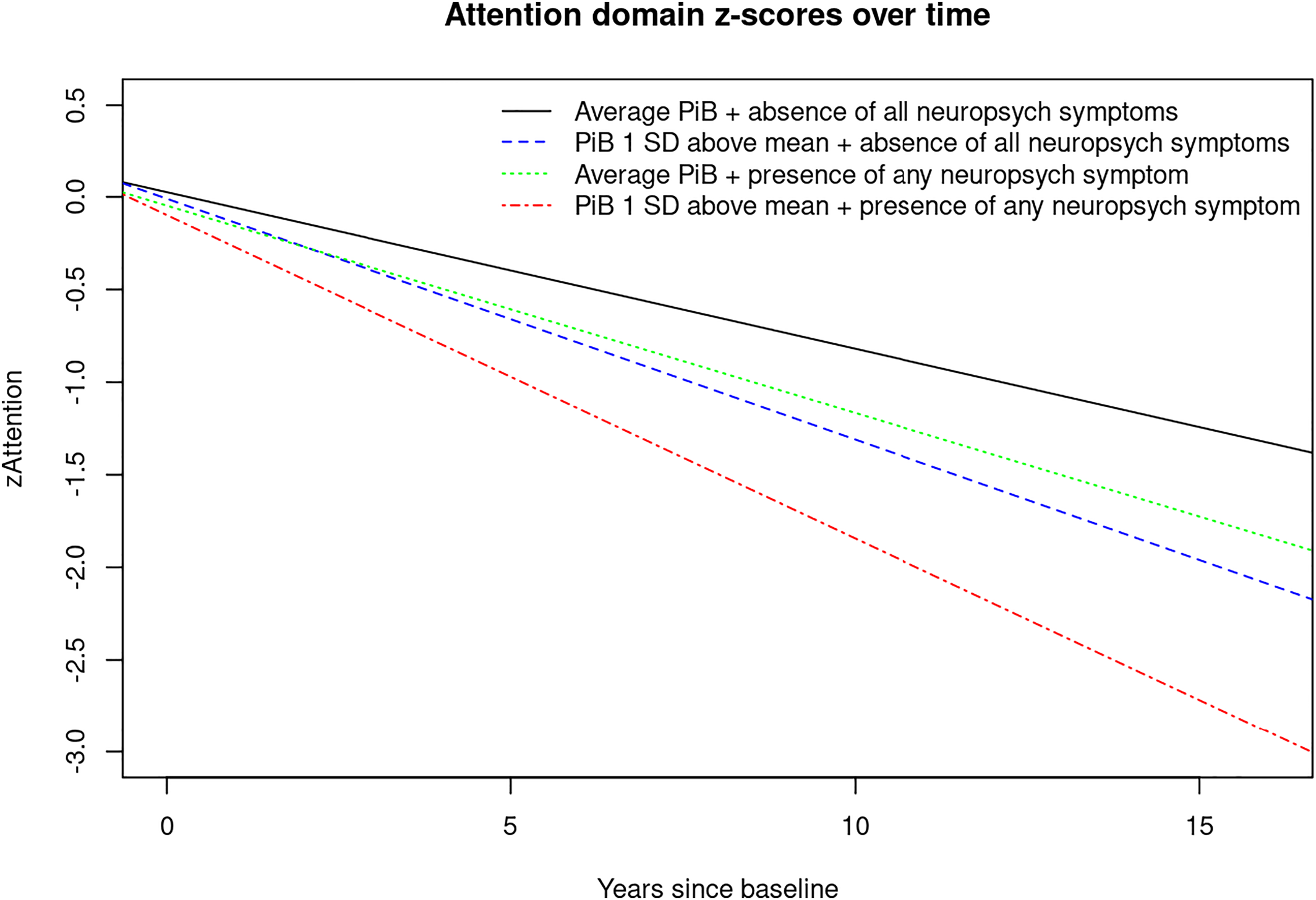
FIGURE 1. Plot of linear mixed effects model for PiB‐PET‐SUVR (average vs. 1 SD above the mean) and presence of any neuropsychiatric symptoms (Yes/No) predicting attention z‐score. Abbreviations: PiB, brain amyloid deposition as measured by PiB‐PET; SD, standard deviation; zAttention, Attention z‐score.
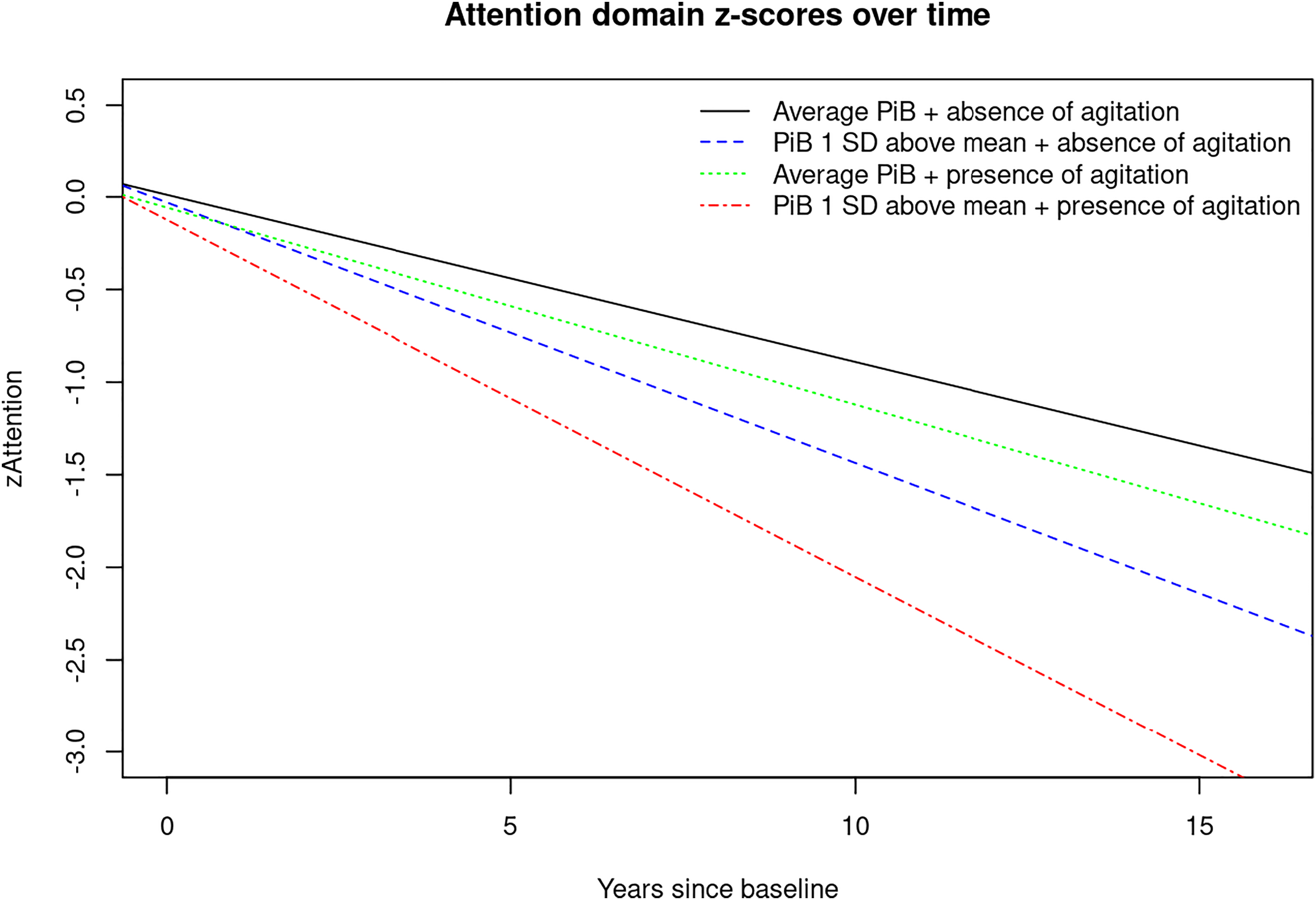
FIGURE 2. Plot of linear mixed effects model for PiB‐PET‐SUVR (average vs. 1 SD above the mean) and presence of agitation (Yes/No) predicting attention z‐score. Abbreviations: PiB, brain amyloid deposition as measured by PiB‐PET; SD, standard deviation; zAttention, Attention z‐score.
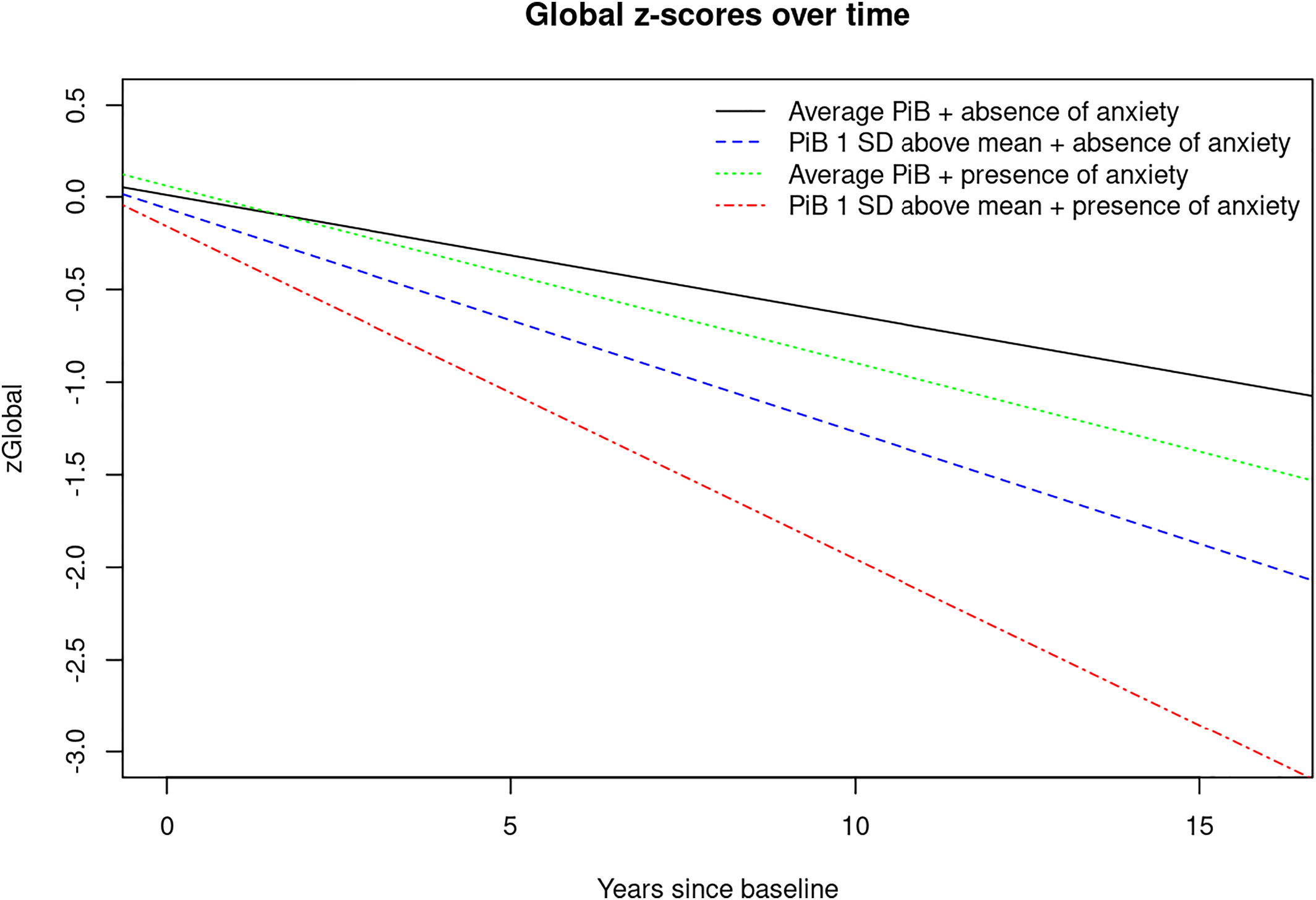
FIGURE 3. Plot of linear mixed effects model for PiB‐PET‐SUVR (average vs. 1 SD above the mean) and presence of anxiety (Yes/No) predicting global cognition z‐score. Abbreviations: PiB, brain amyloid deposition as measured by PiB‐PET; SD, standard deviation; zGlobal, global cognition z‐score.
RESULTS
Demographics
We included 1581 CU community‐dwelling older adults. One thousand one hundred and twenty‐one (70.9%) were PiB‐PET‐, and 460 (29.1%) were PiB‐PET+; 1139 (72.0%) were N‐, and 442 (28.0%) were N+. The mean (SD) age was 71.1 (9.84) years, 54.0% were males, the mean (SD) education was 14.9 (2.59) years and 27.5% were APOEɛ4 carriers. The complete demographic characteristics are summarized in Table 1.
| Variable | Total (N = 1581) |
|---|---|
| Age in years, mean (SD) [range] | 71.1 (9.84) [50.20–95.12] |
| Males | 854 (54.0) |
| Education in years, mean (SD) | 14.9 (2.59) |
| APOE Ɛ4 carrier | 425 (27.5) |
| PiB‐PET SUVR, mean (SD) | 1.5 (0.33) |
| PiB‐PET+ | 460 (29.1) |
| FDG‐PET SUVR, mean (SD) | 1.6 (0.14) |
| N+ | 442 (28.0) |
| Agitation | 28 (1.8) |
| Anxiety | 69 (4.4) |
| Apathy | 63 (4.0) |
| Appetite change | 47 (3.0) |
| Nighttime behaviorb | 72 (5.1) |
| Delusions | 1 (0.1) |
| Depression | 168 (10.6) |
| Disinhibition | 12 (0.8) |
| Euphoria | 7 (0.4) |
| Hallucinations | 1 (0.1) |
| Irritability | 109 (6.9) |
| Motor behavior | 13 (0.8) |
| Any NPS | 332 (21.0) |
| Sum of the 12 NPI‐Q severity scores; 0–36, mean (SD) | 0.5 (1.38) |
| Converted to MCI/dementia during follow‐up | 246 (15.6) |
| 1 visit | 171 (10.8) |
| 2 visits | 168 (10.6) |
| 3 visits | 136 (8.6) |
| 4 visits | 140 (8.9) |
| 5 visits | 167 (10.6) |
| 6 visits | 229 (14.5) |
| 7 visits | 233 (14.7) |
| 8 visits | 164 (10.4) |
| 9 visits | 119 (7.5) |
| 10 visits | 45 (2.8) |
| 11 visits | 6 (0.4) |
| 12 visits | 1 (0.1) |
| 13 visits | 2 (0.1) |
Interactions of Amyloid positivity and Glucose Hypometabolism with Neuropsychiatric Symptoms in predicting Cognitive Decline
Interactions between biomarkers, that is, PiB and NPS in predicting cognitive decline were examined through linear‐mixed effects models (Tables 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15).
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.06521 | −0.07146 | −0.05896 | <0.0001 |
| Time * z‐score PiB | −0.05545 | −0.06204 | −0.04886 | <0.0001 |
| Time * anxiety | −0.03040 | −0.05884 | −0.00195 | 0.0362 |
| Time*z‐score PiB* anxiety | −0.02856 | −0.05464 | −0.00248 | 0.0318 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.06567 | −0.07200 | −0.05933 | <0.0001 |
| Time * z‐score PiB | −0.05561 | −0.06223 | −0.04899 | <0.0001 |
| Time * irritability | −0.01673 | −0.03979 | 0.006341 | 0.1552 |
| Time*z‐score PiB* irritability | −0.02721 | −0.05174 | −0.00268 | 0.0297 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.08454 | −0.09213 | −0. 07,696 | <0.0001 |
| Time * z‐score PiB | −0.04548 | −0.05405 | −0.03691 | <0.0001 |
| Time * any NPS | −0.02735 | −0.04387 | −0.01083 | 0.0012 |
| Time*z‐score PiB* any NPS | −0.01724 | −0.03269 | −0.00180 | 0.0286 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.08703 | −0.09423 | −0.07983 | <0.0001 |
| Time * z‐score PiB | −0.04750 | −0.05517 | −0.03983 | <0.0001 |
| Time * NPS severity | −0.00672 | −0.01155 | −0.00189 | 0.0064 |
| Time*z‐score PiB* NPS severity | −0.00504 | −0.00887 | −0.00120 | 0.0101 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.09028 | −0.09722 | −0.08334 | <0.0001 |
| Time * z‐score PiB | −0.05044 | −0.05776 | −0.04311 | <0.0001 |
| Time * agitation | −0.01613 | −0.06951 | 0.03726 | 0.5538 |
| Time*z‐score PiB* agitation | −0.03609 | −0.07175 | −0.00042 | 0.0473 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.08969 | −0.09666 | −0.08273 | <0.0001 |
| Time * z‐score PiB | −0.04944 | −0.05679 | −0.04209 | <0.0001 |
| Time * appetite | −0.02161 | −0.06072 | 0.01751 | 0.2789 |
| Time*z‐score PiB* appetite | −0.04345 | −0.07470 | −0.01219 | 0.0065 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.09011 | −0.09695 | −0.08328 | <0.0001 |
| Time * z‐score PiB | −0.05120 | −0.05831 | −0.04409 | <0.0001 |
| Time * euphoria | −0.1215 | −0.2247 | −0.01821 | 0.0211 |
| Time*z‐score PiB* euphoria | −0.1495 | −0.2575 | −0.04140 | 0.0067 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.08816 | −0.09523 | −0.08109 | <0.0001 |
| Time * z‐score PiB | −0.04892 | −0.05627 | −0.04157 | <0.0001 |
| Time * irritability | −0.03885 | −0.06445 | −0.01324 | 0.0029 |
| Time*z‐score PiB* irritability | −0.04956 | −0.07828 | −0.02084 | 0.0007 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.04263 | −0.04909 | −0.03616 | <0.0001 |
| Time * z‐score PiB | −0.04887 | −0.05561 | −0.04213 | <0.0001 |
| Time * anxiety | −0.02832 | −0.05759 | 0.000939 | 0.0578 |
| Time*z‐score PiB* anxiety | −0.04337 | −0.06990 | −0.01684 | 0.0014 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.04100 | −0.04763 | −0.03436 | <0.0001 |
| Time * z‐score PiB | −0.04891 | −0.05579 | −0.04202 | <0.0001 |
| Time * depression | −0.03109 | −0.05144 | −0.01073 | 0.0028 |
| Time*z‐score PiB* depression | −0.02623 | −0.04735 | −0.00511 | 0.0150 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.04371 | −0.05008 | −0.03735 | <0.0001 |
| Time * z‐score PiB | −0.05153 | −0.05807 | −0.04499 | <0.0001 |
| Time * motor behavior | −0.09548 | −0.1656 | −0.02540 | 0.0076 |
| Time*z‐score PiB* motor behavior | −0.1023 | −0.1930 | −0.01152 | 0.0272 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.05721 | −0.06383 | −0.05059 | <0.0001 |
| Time * z‐score PiB | −0.05154 | −0.05831 | −0.04476 | <0.0001 |
| Time * euphoria | −0.07485 | −0.1749 | 0.02524 | 0.1427 |
| Time*z‐score PiB* euphoria | −0.1579 | −0.2551 | −0.06080 | 0.0014 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.02353 | −0.02880 | −0.01826 | <0.0001 |
| Time * z‐score PiB | −0.02755 | −0.03295 | −0.02214 | <0.0001 |
| Time * euphoria | −0.00508 | −0.08549 | 0.07533 | 0.9015 |
| Time*z‐score PiB* euphoria | −0.07986 | −0.1590 | −0.00075 | 0.0479 |
| Estimate | Lower CI | Upper CI | P value | |
|---|---|---|---|---|
| Time | −0.02269 | −0.02801 | −0.01736 | <0.0001 |
| Time * z‐score PiB | −0.02638 | −0.03193 | −0.02084 | <0.0001 |
| Time * appetite change | −0.02540 | −0.05497 | 0.004183 | 0.0924 |
| Time*z‐score PiB* appetite change | −0.02510 | −0.04915 | −0.00104 | 0.0409 |
Our analyses showed that those at the average for PiB‐PET SUVR at baseline without the given NPS tended to decrease over time in all cognitive domains. Two‐way interactions revealed that those with higher PiB‐PET SUVR tended to decrease even faster over time for all cognitive z‐scores. Two‐way interactions also revealed that some NPS are associated with increased decline in cognitive z‐scores over time. Additionally, two‐way interactions showed that glucose hypometabolism was associated with faster cognitive decline (Supplemental Material S1).
Most interesting though, are the three‐way interactions we observed. There were significant interactions between years since baseline, increased PiB‐PET SUVR and anxiety or irritability indicating accelerated decline on global cognitive z‐scores, and between years since baseline, increased PiB‐PET SUVR and several NPS indicating faster domain‐specific decline, especially on the attention domain. For example, there were three‐way interactions between years since baseline, increased PiB‐PET SUVR and agitation, appetite change, euphoria, irritability, and any NPI‐Q assessed NPS as well as NPS‐severity showing accelerated decline in attention z‐scores (Tables 4, 5, 6, 7, 8, 9).
Models including PiB‐PET SUVR and NPS in predicting memory z‐scores showed that those with increased PiB‐PET SUVR and anxiety, depression or aberrant motor behavior declined faster (Tables 10, 11, 12). Additionally, there were few interactions between PiB‐PET SUVR and NPS in predicting longitudinal visuospatial and language z‐scores (Tables 13, 14, 15).
These results tell us that NPS can give us extra information beyond PiB‐PET for predicting cognitive decline.
For example, two‐way interactions showed, that participants with any NPS tended to decrease faster over time in attention z‐scores (β [95% CI], −0.0274 [−0.0439, −0.0108], P = 0.0012); and participants with higher PiB‐PET SUVR tended to decrease faster over time in attention z‐scores (−0.0455 [−0.0541, −0.0369], P < 0.001). Furthermore, three‐way interactions revealed that those with increased PiB‐PET SUVR and any NPS declined even faster in the attention domain (−0.0172, [−0.0327, −0.0018], P = 0.0286) (Table 2).
However, we did not observe significant three‐way interactions between years since baseline, lower FDG‐PET‐SUVR and NPS in predicting cognitive z‐score trajectory in our sample of CU. Interactions between FDG‐PET SUVR and NPS were only significant in a sample that also included cognitively impaired individuals (i.e., MCI and dementia) (data not shown).
DISCUSSION
Here we report interactions (two‐way and three‐way) between NPS, PiB and time since baseline with accelerated cognitive decline. Most novel to the present study are the three‐way interactions we observed showing that having NPS and elevated brain amyloid deposition are associated with even further accelerated global and domain‐specific cognitive decline, especially in the attention/executive function domain. For example, there were interactions between time since baseline, PiB, and agitation, appetite change, euphoria, irritability, any NPI‐Q assessed NPS and NPS‐severity indicating faster decline on attention z‐scores. We also observed interactions between time since baseline, PiB, and anxiety, depression and aberrant motor behavior with accelerated decline on memory z‐scores and a few three‐way interactions that reveal accelerated decline on visuospatial and language z‐scores.
While NPS and neuroimaging biomarkers have been shown to be independent predictors of cognitive decline, little is known about the underlying etiologic mechanisms. Our team has previously proposed four possible theoretical explanations for the link between NPS and cogitive decline (41). For example, the etiologic pathway, meaning that NPS may have a direct deleterious effect on the brain leading to cognitive decline. Further theoretical constructs are the shared risk factor or confounding pathway, reverse causality and synergistic interaction. In the current study, we examined the theory of synergistic interaction. Thus, we examined the possibility of NPS interacting with AD pathology, as measured by PiB‐PET, in accelerating cognitive decline.
While several studies have reported associations between NPS and brain amyloid deposition (42, 43, 44, 45) as well as glucose hypometabolism (46, 47), few have examined associations between multi‐modal amyloid and synaptic imaging with cognitive outcomes. For example, we and others have observed that clinically relevant anxiety interacts with amyloid pathology in predicting cognitive decline (16, 17). In the current study we observed interactions between PiB and NPI‐Q assessed anxiety symptoms in accelerating cognitive decline on global cognition and in the memory domain.
When it comes to depression, previous studies have observed longitudinal associations between amyloid imaging and depression (45, 48). Investigators from the Harvard Aging Brain Study have also examined cognitive outcomes and reported a significant interaction between baseline amyloid deposition with higher depressive symptoms on cognitive decline (49). While we previously found that CU persons with both depression (as measured by BDI‐II) and PiB+ were at increased risk of developing MCI, we did not observe significant interactions between these risk factors in predicting MCI (16). In the current study, we observed interactions between NPI‐Q‐assessed depressive symptoms and amyloid deposition in predicting faster cognitive decline in terms of memory.
While findings on depression and anxiety seem to be inconsistent in the previous literature, in the current study, we observed significant interactions between elevated PiB and anxiety with accelerated decline on global cognition and the memory domain; PiB and depression interacted to accelerate decline in the memory domain. However, one should keep in mind the differences in methodology across various studies. For example, it should be noted that in this study we examined CU individuals; participants with cognitive impairment at baseline, (i.e., MCI or dementia) were excluded. Furthermore, the outcome of interest for our analyses was cognitive z‐scores and not clinical syndromes like MCI or dementia. In addition, current research suggests that NPS can fluctuate and unlike cognition do not necessarily proceed in a straight line in a single direction.
To our knowledge, studies investigating interactions between amyloid deposition and a broad spectrum of NPS in accelerating cognitive decline in community‐dwelling individuals are lacking.
While in the current study we found three‐way interactions between PiB‐PET, NPS, and time since baseline in predicting cognitive z‐scores, we did not observe significant three‐way interactions between FDG‐PET, NPS, and time since baseline in predicting the same outcomes. However, it should be noted that while PiB‐PET can identify individuals on the AD‐spectrum even before cognitive decline occurs, FDG‐PET reflects neurodegeneration which is not necessarily specific for AD (14). In this analysis we included only CU at baseline. When we conducted the same analysis in a sample that also included cognitively impaired individuals (i.e. MCI and dementia), we found significant three‐way interactions between neurodegeneration, that is, lower FDG‐PET, various NPS, and time since baseline indicating faster decline on attention z‐scores (data not shown). In addition, our team previously observed that the combined presence of FDG‐PET and NPS increased the risk of incident MCI (50). However, as mentioned above, the differences in study methodology should be noted.
The strengths of our study include the large population‐based sample of CU older adults, and a relatively long follow‐up time of 6.2 years. Furthermore, we examined a broad spectrum of NPS.
Our study also has limitations. Some of the NPI‐Q assessed NPS were rare, thus potentially limiting statistical power (e.g., only one participant had delusions, seven had euphoria, and one had hallucinations). However, considering that our study sample consisted of community‐dwelling persons, low numbers in some strata are expected. Furthermore, data on nighttime behavior was missing for 164 participants. In addition, we did not adjust for multiplicity of testing which could be interpreted as a limitation by some. However, it is also important to note that some investigators do not recommend Bonferroni correction to avoid type 2 error. Also, in light of the previous literature, the results seem plausible and build a foundation to understanding and examining potential mechanisms that may underly the association between NPS and AD. Furthermore, our sample is relatively highly educated and 98% of study participants are of Caucasian decent. However, it has been shown that data from Olmsted County are generalizable to the U.S. population of Minnesota and the Upper Midwest (51), even though, generalization to ethnic minorities is still limited.
In summary, our study shows that NPS can give us extra information beyond PiB‐PET for predicting faster global and domain‐specific cognitive decline, especially in the attention domain. Based on our findings, it is possible that NPS interact with AD pathology, as measured by PiB‐PET, in accelerating global and domain specific cognitive decline in CU community‐dwelling older adults.
Furthermore, this study emphasizes the importance of NPS in AD‐research, and underlines the clinical importance of NPS in the early stages of AD, that is, individuals on the AD spectrum without cognitive impairment. More studies are needed to confirm our findings.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51



