Progression of Bilateral Striopallidal Calcinosis and Parkinsonism in a Case of Gorlin Syndrome
To the Editor: Gorlin syndrome 1 is a rare autosomal dominant inherited phacomatosis with an estimated prevalence of 1 in 57,000 to 1 in 164,000. But nearly 50% of all cases are new mutations. Typical findings in Gorlin syndrome include basal cell carcinomas, odontogenic keratocysts, palmar and plantar pits, and calcification of the falx cerebri. 2 Intracranial calcification of the tentorium cerebelli, petroclinoid ligaments, dura, pia, choroids plexus and basal ganglia have been described. 3 However these cases did not show associated clinical symptoms. We report a case of Gorlin syndrome with bilateral striopallidal calcinosis and progressive parkinsonism.
Case Report
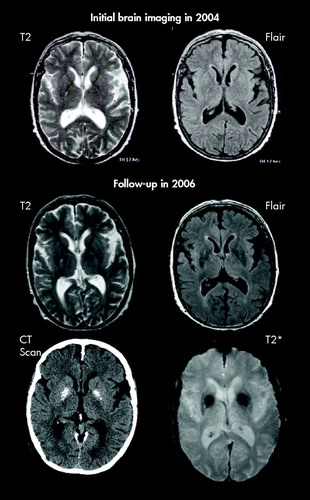
A 70-year-old woman with Gorlin syndrome was treated with chlorpromazine and fluphenazine for delusion and depression since the age of 30. While during more than 35 years of antipsychotic treatment no features of extrapyramidal disease were seen, she developed a hypokinetic rigid syndrome with tremor in 2004. Antipsychotic medication was switched first to 5 mg olanzapine and then to 50 mg quetiapine, but parkinsonian features remained unchanged (UPDRS III score 44). In 2004, a brain MRI showed no abnormality of the basal ganglia ( Figure 1 ). l -dopa-response was tested in 2006 and not only found to be negative but also led to vivid dreams and delusions. Follow-up brain MRI (T2, Flair, and T2*) and a CT scan in 2006 showed two 1.5 cm symmetrical striopallidal calcinosis. As serum calcium and parathormone levels were normal, hypoparathyroidism could be excluded. Neither the Mini-Mental State Examination nor the clinical observation pointed to memory impairment.
Discussion
Since the introduction of CT basal ganglia calcification has been described in otherwise healthy elderly individuals and in symptomatic patients. This led to an ongoing discussion about significance and relationship between clinical, radiological, or pathological findings in basal ganglia calcifications. Incidence of basal ganglia calcification was reported to be about 6.6 per 1,000 in a screening of CT scans. In most patients calcification was rather small and usually confined to the globus pallidus. In the symptomatic patients the amount of calcification was significantly greater than in asymptomatic patients. 4 Our patient developed parallel parkinsonism and a quite extensive striopallidal calcinosis over 2 years. The simultaneous development of calcifications and clinical symptoms points to a correlation between calcification and parkinsonism, although an influence of long-term antipsychotic treatment cannot be ruled out completely. Drug-induced parkinsonism, however, is in most cases reversible and resolves after the medication is stopped or switched to quetiapine. Tardive dsykinesia is characterized by coordinated, constant movements of the mouth, tongue, jaw, and cheeks. If trunk movements are present, they are typically found in the form of rapid forward motions of the lower abdomen and hips or twisting or flicking movements of the arms and legs. None of this was present. To avoid the misleading name of “Fahr`s disease,” a new classification which considers the whole spectrum of disorders in which basal ganglia calcification can be found was proposed in 2005. 4 Within this classification our case meets criteria for bilateral striopallidal calcinosis. In a recent study about radiological features of Gorlin syndrome 2% of the patients showed basal ganglia calcification. 3 Basal ganglia calcification in Gorlin syndrome has to be considered as a rare feature in a rare disease. In our case calcification occurred at 68 years old. Medium age in the above mentioned study was 35 years old, and these patients were screened only once by MRI or CT. All affected patients were discovered in the CT group. As intracranial calcification is associated with Gorlin syndrome we would recommend using CT and an MRI protocol including susceptibility-weighted MRI sequences (T2*). This combination appears useful in order to visualize calcifications and other potential pathology accountable for the clinical features with high sensitivity. 5 When clinically indicated, follow-up MRI may be very useful in demonstrating the progression of pathological changes. This may provide a background for the understanding of clinical progression and unfavorable response to therapy in l -dopa-resistant atypical Parkinson’s syndrome.
1. Gorlin RJ, Goltz RW: Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med 1960; 262:908–912Google Scholar
2. Gorlin RJ: Nevoid basal cell carcinoma syndrome. Dermatol Clin 1995; 13:113–125Google Scholar
3. Kimonis VE, Mehta SG, Digiovanna JJ, et al: Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. Genet Med 2004; 6:495–502Google Scholar
4. Manyam BV: What is and what is not “Fahr’s disease.” Parkinsonism Relat Disord 2005; 11:73–80Google Scholar
5. Bottcher J, Sauner D, Jentsch A, et al: [Visualization of symmetric striopallidodentate calcinosis by using high-resolution susceptibility-weighted MR imaging. An account of the impact of different diagnostic methods of M Fahr.] Nervenarzt 2004; 75:355–361 (German)Google Scholar



