Generalized and Symptom-Specific Insight in Behavioral Variant Frontotemporal Dementia and Primary Progressive Aphasia
With increasing disease duration, the symptom profiles of these two related dementias become less distinct: symptoms of aphasia can emerge later in the course of behavioral variant FTD, 8 and PPA patients often become behaviorally more similar to behavioral variant FTD patients. 7 Few studies have assessed insight in PPA, but evidence does point to loss of insight into select symptoms in this disorder. This is consistent with evidence from other neurological diseases, which demonstrate that loss of insight is sometimes quite radical, with a total denial of a particular symptom, but in other cases insight can be only partially diminished. 1 , 9 The symptoms seen in dementias caused by FTLD can be grossly divided into two categories: behavioral symptoms such as disinhibition, apathy, or lack of spontaneity, and cognitive symptoms such as aphasia or difficulties with attention or executive functions. In the frontotemporal dementias and other disorders, including Alzheimer’s disease, studies suggest that behavioral symptoms are more vulnerable to loss of insight than certain cognitive symptoms. 10 , 11 In PPA, language is the most affected domain. Few studies have assessed insight into language symptoms, although the evidence points to reduced insight in some patients with PPA, 12 with behavioral symptoms such as apathy most often associated with reduced insight. 10
Levels of insight can be assessed by administering identical questionnaires to patients and their caregivers and calculating the discrepancy between their responses. This method has been applied to the study of insight into particular, isolated symptoms such as empathy 13 and disinhibition. 14 However, this technique has rarely been used to compare insight across a range of symptoms in a particular disease. The current study compared patient and caregiver ratings on a measure specifically designed to capture the spectrum of symptoms seen in FTLD, the Frontal Behavioral Inventory. 15 Questions on the Frontal Behavioral Inventory survey cognitive symptoms, including aphasia and inattention, and behavioral symptoms, including apathy and disinhibition. Assuming that the caregiver has more objectivity than the patient, this method permits the evaluation of patient insight into disease-related changes by comparing the total Frontal Behavioral Inventory scores in both groups. In addition, insight into specific symptoms can be investigated by comparing caregiver and patient scores on individual items.
The aims of this study were twofold. The first was to compare patient and caregiver scores on the Frontal Behavioral Inventory, with the hypothesis that preserved insight in the PPA group would be reflected in a smaller discrepancy between patient and caregiver scores than in the behavioral variant FTD group. The second aim was to assess symptom-specific insight by comparing the discrepancy between patient and caregiver scores on individual items on the Frontal Behavioral Inventory. For all groups, it was predicted that behavioral symptoms would be associated with greater discrepancy in symptom scores than cognitive symptoms.
METHODS
Participants
Patients with behavioral variant FTD, PPA, and probable Alzheimer’s disease with similar disease duration were recruited for this study from the Clinical Core of the Northwestern Alzheimer’s Disease Center. We obtained informed consent from each patient and his or her caregiver on a protocol approved by the Institutional Review Board at Northwestern University. The most recent research consensus criteria were used for diagnosis, 4 , 16 , 17 and diagnoses were made via consensus among a team of behavioral neurologists and neuropsychologists. The Mesulam criteria used to diagnose PPA do not subtype further, although other groups use the Neary criteria and subtype into semantic dementia and progressive nonfluent aphasia. Our study employed the Mesulam criteria exclusively, and hence all patients with 2 years of relatively isolated aphasia, in addition to the other criteria, were included. This group included patients with varying degrees of fluency in their output, as well as variation in comprehension deficits and agrammatism. Although the Neary criteria stipulate loss of insight as a core criterion for behavioral variant FTD, this criterion is open to interpretation. 5 Patients with behavioral variant FTD in the current study were judged clinically to have reduced insight into some aspect of their disease and hence met diagnostic criteria. The diagnostic criteria for PPA do not specify a required level of insight. The probable Alzheimer’s disease group was included as a comparison group, since insight has been well researched 11 , 18 – 20 and is better understood in this condition. Only those patients who were able to complete a clinical neuropsychological evaluation and were clinically judged to have adequate language comprehension to complete the study were included. In addition, only patients with mild or moderate levels of dementia, as determined by a Mini-Mental State Examination (MMSE 21 ) score greater than or equal to 10 and a Clinical Dementia Rating scale (CDR 22 , 23 ) global score less than or equal to 2, were included. Demographic and disease-severity information is summarized in Table 1 .
 |
Procedures
The Frontal Behavioral Inventory was developed as a structured caregiver interview that specifically targets common symptoms in FTLD and has been shown to have strong psychometric properties. 15 , 24 The version used in our current study was slightly modified by the original author, who standardized it and found it to be equivalent to the earlier versions (Kertesz, personal communication, 2004). One question per symptom is asked, with a total of 24 questions. Half of the questions address “negative” symptoms such as apathy and withdrawal, and half of the questions address “positive” symptoms such as disinhibition. Each symptom is rated on a Likert scale ranging from 0 (none/never) to 3 (severe/always). In this study, the Frontal Behavioral Inventory was given to both the caregiver and the patient in the course of separate structured interviews. Each of the examiners who completed the questionnaires with the participants was trained by the first author to establish that the participants received the same information and instructions.
Data Analysis
The Frontal Behavioral Inventory discrepancy scores were calculated by subtracting the total caregiver score from the total patient score. Since a higher Frontal Behavioral Inventory score implies more, or more severe, symptoms, a negative discrepancy score indicates loss of insight; the more negative the discrepancy, the less insight. The Frontal Behavioral Inventory discrepancy scores for each of the subgroups were not normally distributed, and large differences existed between the variance of scores in each subgroup. Given these violations of the assumptions necessary for parametric comparisons, in addition to the ordinal nature of the data, nonparametric statistics were employed. Total discrepancy scores were compared among the three groups using the Kruskal-Wallis test and between groups with Mann-Whitney tests, with a Bonferroni correction (a lowered alpha of 0.017 was accepted as significant).
To assess symptom-specific insight, patient-caregiver discrepancy scores for individual items on the Frontal Behavioral Inventory were identified for item analysis. In order to focus the analysis on symptoms which occurred with adequate frequency in both PPA and behavioral variant FTD groups, and to avoid excessive numbers of comparisons, the caregiver responses were examined to exclude items that were rarely endorsed. Caregiver responses were then ranked and the seven most commonly endorsed symptoms were picked for further analysis, excluding all other items from further analysis to avoid a potential increase in type I error. Details of item selection are explained in the Results section. The individual symptom discrepancy scores for these items (patient’s symptom score minus caregiver’s symptom score) were then calculated. Wilcoxon signed ranks test was used to test whether patient and caregiver scores differed significantly for each symptom. All tests were two-tailed.
RESULTS
First, overall differences in insight among groups were compared using the Frontal Behavioral Inventory discrepancy score. Figure 1 shows the distribution of scores. There was a significant effect of diagnosis (H=18.26, df=2, p<0.0005). Comparisons between groups indicated that the PPA patients’ discrepancy scores were significantly closer to zero, i.e., better patient-caregiver agreement than the behavioral variant FTD (U=9, p<0.0005) and the probable Alzheimer’s disease (U=61, p<0.0005) groups. The probable Alzheimer’s disease and behavioral variant FTD groups did not differ significantly. Data were also analyzed by dividing both the patient and caregiver total Frontal Behavioral Inventory scores by the number of symptoms that the caregiver endorsed, then comparing the difference between patient and caregiver scores within each of the groups. This step eliminates any bias resulting from the larger number of symptoms that behavioral variant FTD patients typically demonstrate on the Frontal Behavioral Inventory. 15 The same pattern found in the original analysis was evident (significant effect of diagnosis [H=19.15, df=2, p<0.0005]). There was no difference between the Alzheimer’s disease and behavioral variant FTD groups, and the PPA patients showed better agreement with their caregivers relative to the Alzheimer’s disease group (U=51.5, p<0.0005) and the behavioral variant FTD group (U=10.0, p<0.0005). In addition, given the wide variance in scores seen in each of the groups, correlations were performed with various disease-severity measures (MMSE, CDR, Activities of Daily Living Questionnaire 25 ) for each of the groups. None of these correlations were significant.

PPA=primary progressive aphasia; FTD=frontotemporal dementia
Subsequent to these analyses, symptoms were ranked in terms of the frequency with which they were endorsed by caregivers in order to select symptoms that would be used for symptom-specific analysis. Overall, symptoms were less frequently endorsed by PPA caregivers than by behavioral variant FTD caregivers. Therefore, the seven symptoms most frequently endorsed by PPA caregivers were selected. The frequency of endorsement and the ranking of these symptoms for the groups are presented in Table 2 .
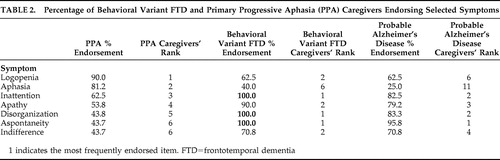 |
Results of the Wilcoxon signed ranks test on the Frontal Behavioral Inventory symptom discrepancy scores (patient score minus caregiver score) for each of the seven symptoms is displayed in Table 3 . The scores indicated that the PPA group demonstrated significant differences between caregiver and patient responses only for apathy and aspontaneity, the behavioral variant FTD group differed significantly on all symptoms except aphasia, and the probable Alzheimer’s disease group differed significantly for all symptoms except aphasia.
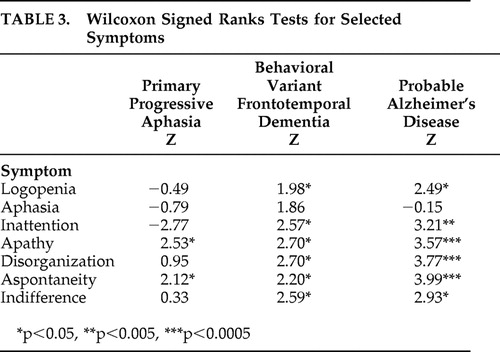 |
DISCUSSION
Behavioral variant FTD patients were found to disagree with their caregivers more than PPA patients on their overall symptom frequency and intensity. However, there was substantial variability among patients in the behavioral variant FTD group, with some having similar levels of insight as PPA patients. Patients with PPA were more likely to be in closer agreement with their caregivers. There was also a relatively wide range of discrepancy scores within the PPA group, suggesting that some patients do, in fact, demonstrate poor insight. The comparison group of probable Alzheimer’s disease patients did not differ significantly from the behavioral variant FTD patients and showed the greatest variability in the degree of loss of insight. When symptom-specific insight was analyzed, behavioral variant FTD and probable Alzheimer’s disease patients disagreed with their caregivers for all symptoms analyzed except aphasia. Patients with PPA, however, disagreed with their caregivers only for apathy and aspontaneity. Patients with PPA tended to rate their language and other cognitive symptoms similarly to their caregivers’ ratings.
The majority of behavioral variant FTD patients in this study showed poor insight. However, there was a wide range of discrepancy on Frontal Behavioral Inventory scores in this group. The finding that some patients with behavioral variant FTD may have relatively intact insight was not expected, given that loss of insight is a core diagnostic criterion for behavioral variant FTD by Neary criteria. 4 This finding echoes that of a recent study investigating the empirical basis of this criterion. 5 Evers et al. 5 found three of eight behavioral variant FTD patients have intact insight when they used a semi-structured interview method directly asking patients about their disease. Another study found that even when behavioral variant FTD patients acknowledged their behavioral symptoms, they still did not express concern about how these symptoms might affect them or their families. 26 It may be that those behavioral variant FTD patients who were in closer agreement to their caregivers in the current study also demonstrated this pattern of anosodiaphoria (lack of concern regarding symptoms 27 ) as opposed to a frank anosognosia.
Another surprising finding was the lack of significant difference in levels of insight between the behavioral variant FTD and probable Alzheimer’s disease groups. The probable Alzheimer’s disease group also demonstrated the widest range of Frontal Behavioral Inventory discrepancy scores, suggesting that some of these patients had intact insight and others had poor insight. Previous studies regarding insight in probable Alzheimer’s disease also demonstrated variable levels of insight. The involvement of more frontal regions of the brain has been proposed as an explanation for reduced insight in some probable Alzheimer’s disease patients. 28 The lack of association between level of insight and disease severity measures further suggests that insight is not simply lost with progression of disease, but a more complex (likely neuroanatomical) explanation is needed.
The results of this study suggest that behavioral symptoms and non-language cognitive symptoms are more commonly associated with loss of insight than language symptoms. Research in various neurological disorders points to the symptom-specificity of insight. 29 – 31 With probable Alzheimer’s disease patients, Starkstein et al. 20 observed that insight for cognitive symptoms is distinct from insight for behavioral symptoms, and others have reported that loss of insight is commonly associated with behavioral symptoms in dementias caused by FTLD. 6 In our study, the behavioral variant FTD and probable Alzheimer’s disease patients behaved in a very similar manner in terms of symptom-specific insight, whereas PPA patients lost insight only into apathy and aspontaneity, which are behavioral symptoms, albeit closely related. Eslinger et al. 6 suggested that apathy and aspects of empathy were particularly sensitive to loss of insight in both behavioral variant FTD (they labeled these patients “Social/Dysexecutive” subtype) and the semantic dementia subtype of PPA; however, their behavioral variant FTD group differed from their PPA group in terms of insight into cognitive symptoms. The authors also found that behavioral variant FTD patients showed poor insight into cognitive symptoms such as memory and attention, whereas PPA patients complained of cognitive symptoms to a similar degree as their caregivers.
Numerous studies in probable Alzheimer’s disease have suggested that loss of insight into apathy is particularly common. 20 , 32 It could be that apathy and insight are not, in fact, dissociable but that the lack of concern seen in apathy is conceptually related to anosognosia or, at least, anosodiaphoria. Attempts to treat apathy in neurological and psychiatric diseases with various medications have had mixed, but sometimes encouraging, results. 33 – 35 If insight and apathy are strongly intertwined, successfully reducing apathy with medication may affect insight. This concept has yet to be explored.
Some of the symptoms investigated (e.g., apathy, indifference) may also reflect mood disturbances, which have proved to be common in PPA 36 and Alzheimer’s disease 37 but not to the same degree in behavioral variant FTD. 38 These mood disturbances may relate to an increased rate of anosodiaphoria or may also disturb a mechanism of insight—somatic “tagging” 39 of information by the emotion network—making the information (in this case, about disease symptoms) more salient. Further research into this area is warranted.
Insight into aphasia was intact even in the behavioral variant FTD and probable Alzheimer’s disease groups whereas loss of insight for other cognitive symptoms was common. This is interesting in light of the underlying neuroanatomy. Aphasia is associated with damage to the left hemisphere perisylvian language region. Anosognosia, for the most part, is considered to be a right hemisphere phenomenon. 1 , 40 Aphasia may in some way be “protected” from loss of insight due to the hemispheric localization of language. However, some patients with Wernicke’s aphasia appear to show loss of insight into their disordered speech despite the left hemisphere locus of damage. 41 , 42 Those PPA patients who show reduced insight produce language with reduced meaningful content, similar to Wernicke’s patients. 12 However, it has yet to be established whether patients ever lose insight specifically into their language symptoms. The relationship between loss of insight in dementia and language symptoms warrants further investigation.
The lack of insight for even certain cognitive symptoms in behavioral variant FTD is consistent with findings in the literature that these patients lose their sense of “selfhood.” 43 It is possible that they no longer have a good sense of how they used to be, and hence they are unable to realistically compare their ability now to their ability before their disease onset. Rankin et al. 13 have revealed a tendency for behavioral variant FTD patients to overestimate positive aspects of their personalities such as assuredness and extroversion, while underestimating negative qualities such as cold-heartedness. It could be argued that behavioral variant FTD patients similarly hold a delusional belief that they are not impaired. During the disease process of behavioral variant FTD, patients may not passively lose awareness into their symptoms, but actively develop a new, positive self-view. Results from the current study suggest that PPA patients, in contrast, will sometimes actually be more critical of their abilities than their caregivers, potentially related to increased levels of depressive symptomatology in these patients. 36
A number of important limitations to this study should be discussed. Our patients were matched in terms of disease duration, but differed on other indices of dementia severity. The Clinical Dementia Rating scale, with its emphasis on memory, may not be as accurate in assessing severity in non-Alzheimer’s dementias as it is in Alzheimer’s disease. Future studies may use other strategies in matching the patient groups. In addition, the patients were relatively homogenous in terms of disease duration, preventing analysis of the impact of this variable on insight. Discrepancy techniques, by definition, rely on the subjective responses of both patients and caregivers, which not only adds between-subject variability but may also be affected by factors other than the patients’ actual symptoms, such as caregiver distress (which has been previously found to be higher when the patient has reduced awareness 44 ) or quality of caregiver observation. This technique also fails to establish what aspect of insight is failing, for example whether there is a breakdown in general self-awareness or a more specific self-monitoring deficit (for discussions of these components of insight, see references 10 , 29 , 45 ). Finally, the group sizes, while typical of studies with these less common dementia populations, were not large. This limited the power of the statistical comparisons.
Reduced insight has important clinical implications in terms of caregiver burden, treatment compliance, and prognosis. Better understanding of this phenomenon in dementias caused by frontotemporal lobar degeneration will result in improved caregiver education, which has been shown to be beneficial in informing caregivers and enhancing coping strategies. 46 Currently, quantification of loss of insight is not a common part of the neurological or neuropsychological examination, but it may be an important addition, especially when loss of insight is considered a diagnostic sine qua non for behavioral variant frontotemporal dementia.
1. Prigatano GP: Disturbances of self-awareness of deficit after traumatic brain injury, in Awareness of Deficit After Brain Injury. Edited by Schacter DL, Prigatano GP. New York, Oxford University Press, 1991, pp 111–126Google Scholar
2. Bozzola FG, Gorelick PB, Freels S: Personality changes in Alzheimer’s disease. Arch Neurol 1992; 49:297–300Google Scholar
3. Migliorelli R, Teson A, Sabe L, et al: Anosognosia in Alzheimer’s disease: a study of associated factors. J Neuropsychiatry Clin Neurosci 1995; 7:338–344Google Scholar
4. Neary D, Snowden JS, Gustafson L, et al: Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554Google Scholar
5. Evers K, Kilander L, Lindau M: Insight in frontotemporal dementia: conceptual analysis and empirical evaluation of the consensus criterion “loss of insight” in frontotemporal dementia. Brain Cogn 2007; 63:13–23Google Scholar
6. Eslinger PJ, Dennis K, Moore P, et al: Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2005; 76:1630–1635Google Scholar
7. Marczinski CA, Davidson W, Kertesz A: A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cogn Behav Neurol 2004; 17:185–190Google Scholar
8. Blair M, Marczinski CA, Davis-Faroque N, et al: A longitudinal study of language decline in Alzheimer’s disease and frontotemporal dementia. J Int Neuropsychol Soc 2007; 13:237–245Google Scholar
9. McGlynn SM, Schacter DL: Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol 1989; 11:143–205Google Scholar
10. Banks S, Weintraub S: Self-awareness and self-monitoring of cognitive and behavioral deficits in behavioral variant frontotemporal dementia, primary progressive aphasia and probable Alzheimer’s disease. Brain Cogn 2008; 67:58–68Google Scholar
11. Starkstein SE, Jorge R, Mizrahi R, et al: A diagnostic formulation for anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006; 77:719–725Google Scholar
12. Banks SJ, Weintraub S: Cognitive deficits and reduced insight in primary progressive aphasia. Am J Alzheimers Dis Other Demen 2008; 23:363–371Google Scholar
13. Rankin KP, Baldwin E, Pace-Savitsky C, et al: Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry 2005; 76:632–639Google Scholar
14. Starkstein SE, Garau ML, Cao A: Prevalence and clinical correlates of disinhibition in dementia. Cogn Behav Neurol 2004; 17:139–147Google Scholar
15. Kertesz A, Davidson W, Fox H: Frontal Behavioral Inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997; 24:29–36Google Scholar
16. McKhann G, Drachman D, Folstein M, et al: Clinical Diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Google Scholar
17. Mesulam MM: Primary progressive aphasia. Ann Neurol 2001; 49:425–432Google Scholar
18. Clare L, Wilson BA, Carter G, et al: Awareness in early-stage Alzheimer’s disease: relationship to outcome of cognitive rehabilitation. J Clin Exp Neuropsychol 2004; 26:215–226Google Scholar
19. Dalla Barba G, Parlato V, Iavarone A, et al: Anosognosia, intrusions and “frontal” functions in Alzheimer’s disease and depression. Neuropsychologia 1995; 33:247–259Google Scholar
20. Starkstein SE, Sabe L, Chemerinski E, et al: Two domains of anosognosia in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1996; 61:485–490Google Scholar
21. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975; 12:189–198Google Scholar
22. Hughes CP, Berg L, Danziger WL, et al: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Google Scholar
23. Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Google Scholar
24. Kertesz A, Nadkarni N, Davidson W, et al: The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468Google Scholar
25. Johnson N, Barion A, Rademaker A, et al: The Activities of Daily Living Questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord 2004; 18:223–230Google Scholar
26. Mendez MF, Shapira JS: Loss of insight and functional neuroimaging in frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2005; 17:413–416Google Scholar
27. Critchley M: The Parietal Lobes. London, Edward Arnold, 1953Google Scholar
28. Michon A, Deweer B, Pillon B, et al: Relation of anosognosia to frontal lobe dysfunction in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1994; 57:805–809Google Scholar
29. Barrett AM, Eslinger PJ, Ballentine NH, et al: Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology 2005; 64:693–699Google Scholar
30. Breier JI, Adair JC, Gold M, et al: Dissociation of anosognosia for hemiplegia and aphasia during left-hemisphere anesthesia. Neurology 1995; 45:65–67Google Scholar
31. von Hagen KO, Ives ER: Anosognosia (Babinski) imperception of hemiplegia: report of six cases, one with autopsy. Bulletin of Los Angeles Neurological Society 1937; 2:95–103Google Scholar
32. Ott BR, Noto RB, Fogel BS: Apathy and loss of insight in Alzheimer’s disease: a SPECT imaging study. J Neuropsychiatry Clin Neurosci 1996; 8:41–46Google Scholar
33. Deakin JB, Rahman S, Nestor PJ, et al: Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology 2004; 172:400–408Google Scholar
34. Padala PR, Burke WJ, Bhatia SC, et al: Treatment of apathy with methylphenidate. J Neuropsychiatry Clin Neurosci 2007; 19:81–83Google Scholar
35. Pitkala KH, Laurila JV, Strandberg TE, et al: Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatrics 2004; 16:61–74Google Scholar
36. Medina J, Weintraub S: Depression in primary progressive aphasia. J Geriatr Psychiatry Neurol 2007; 20:153–160Google Scholar
37. Migliorelli R, Teson A, Sabe L, et al: Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry 1995; 152:37–44Google Scholar
38. Banks SJ, Weintraub S: Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J Geriatr Psychiatry Neurol 2008; 21:133–141Google Scholar
39. Bechara A, Damasio H, Damasio AR, et al: Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 1999; 19:5473–5481Google Scholar
40. Heilman KM, Barrett AM, Adair JC: Possible mechanisms of anosognosia: a defect in self-awareness. Philosophical Transactions of The Royal Society of London. Series B, Biological Sciences 1998; 353:1903–1909Google Scholar
41. Shuren JE, Hammond CS, Maher LM, et al: Attention and anosognosia: the case of a jargonaphasic patient with unawareness of language deficit. Neurology 1995; 45:376–378Google Scholar
42. Weinstein EA, Cole M, Mitchell MS, et al: Anosognosia and aphasia. Arch Neurol 1964; 10:376–386Google Scholar
43. Miller BL, Seeley WW, Mychack P, et al: Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 2001; 57:817–821Google Scholar
44. DeBettignies BH, Mahurin RK, Pirozzolo FJ: Insight for impairment in independent living skills in Alzheimer’s disease and multi-infarct dementia. J Clin Exp Neuropsychol 1990; 12:355–363Google Scholar
45. O’Keeffe FM, Murray B, Coen RF, et al: Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain 2007; 130:753–764Google Scholar
46. Banks SJ, Rogalski E, Medina JE, et al: Organizing a series of education and support conferences for caregivers of individuals with frontotemporal dementia and primary progressive aphasia. Alzheimer’s Care Quarterly 2006; 7:243–250Google Scholar



