Neural Correlates of Strategic Processes Underlying Episodic Memory in Women with Major Depression: A 15 O-PET Study
While there is extensive literature characterizing the neuropsychology of the verbal episodic memory impairments found in patients with major depressive disorder, there are only a few neuroimaging studies investigating the neural correlates of these deficits. For example, a study by Bremner and colleagues 9 employed use of PET and a paragraph recall test to evaluate the neural correlates of verbal episodic memory and showed decreased activation of the right anterior cingulate cortex and hippocampus in patients with major depressive disorder relative to healthy comparison subjects. In replication of these findings, De Asis and colleagues 10 also showed reduced activation of the anterior cingulate cortex and hippocampus in patients with major depressive disorder during a task that involved recall of letters presented verbally. 10
In complement to these studies, we evaluate the semantic organizational component of verbal episodic memory in patients with major depressive disorder by employing a modified CVLT paradigm known to induce robust activation of the orbital and lateral prefrontal regions that mediate initiation of semantic processing and organization in healthy comparison subjects. 11 , 12 In terms of the three basic components of memory (i.e., encoding, retention, and retrieval), organizational strategies are believed to be recruited primarily during encoding. In this regard, our paradigm involves presenting word lists with varying degrees of orientation regarding the semantic structure of the list (e.g., instructions versus no instructions about the potential semantic nature of a list) and in varying degrees of organizational processing.
Prior studies that have evaluated depressed patients’ organizational abilities and ability to implement environmental cues have shown impairments of both of these capacities. 13 – 15 Thus, in contrast to healthy comparison subjects who show enhanced recall when a word list contains semantic similarities, and who show further improvement in recall when they are explicitly prewarned of the semantic similarities present in the list (e.g., categories), patients with major depressive disorder might be predicted to show impaired recall when a word list containing semantic similarities is presented either with or without explicit orientation regarding the categorical nature of the list. Memory performance in depressed patients, though, has repeatedly been shown to vary in accord with their degree of depressive symptoms. 4 , 16 Therefore, in our study, we expected to observe a decline in memory performance that correlated with the ratings of depressive severity (i.e., Beck Depression Inventory scores).
In terms of the neuroanatomy underlying the aforementioned functions, regions implicated by prior studies as mediating semantic coding and use of environmental cues include the lateral prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex. Specifically, the orbitofrontal cortex has been implicated in the use of cues for strategy initiation, 11 and the anterior cingulate cortex mediates attentional processes that are required to initiate behaviors. 17 , 18 The dorsolateral and ventrolateral prefrontal cortex have been implicated in semantic organization, 19 , 20 and the anterior cingulate cortex has been shown to mediate category driven word generation. 21
Thus, in terms of neuroimaging hypotheses, for depressed subjects relative to healthy control subjects we predicted that the orbitofrontal cortex and anterior cingulate cortex would show reduced activation during strategy initiation, and the anterior cingulate cortex and either or both aspects of the lateral prefrontal cortex (i.e., the dorsolateral prefrontal cortex and/or ventrolateral prefrontal cortex) would show reduced activation during semantic encoding. In addition, because the hippocampus is also a key structure recruited during encoding 22 , 23 and has shown reduced function in depressed subjects, 10 , 25 , 26 we predicted reduced activation of the hippocampus across all our tasks (in depressed subjects).
METHODS
Participants
Eight women with major depressive disorder and eight healthy women, matched for age, were recruited through the Massachusetts General Hospital Depression and Clinical Research Center. Significant gender differences exist for language and brain function, 24 , 25 and depression may have a gender specific clinical profile, 26 , 27 so our study was limited to female subjects. None of the patients with major depressive disorder was related to persons in the healthy comparison group.
The study protocol was approved by the Massachusetts General Hospital Subcommittee on Human Studies and all participants returned informed written consent. All participants received a structured clinical interview and, 28 except for the diagnosis of major depressive disorder in the patient group, participants were found to be free of axis I psychiatric disorders. On the day of scanning, participants completed the vocabulary, similarities, and information sections of the WAIS-R and the severity of each patient’s depressive symptoms was scored by means of the Beck Depression Inventory (BDI-II). 29 All participants were free of psychotropic medications for at least 2 months prior to scanning. Two patients with major depressive disorder had a diagnosis of hypothyroidism but were taking stable doses of Synthroid and reported having tested euthyroid (i.e., “lab tests” within the normal range, as per patient’s report). Otherwise, neither patients with major depressive disorder nor healthy comparison subjects had a history of medical or neurological disorders. Eighteen participants were scanned. One was eliminated because of excessive movement during scanning and one was eliminated because she fell asleep during scanning.
Experimental Paradigm
This paradigm has been described in detail previously. 11 Briefly, participants underwent eight 60 second oxygen-15 positron emission tomography (PET) scans (each separated by 10 minutes). Two of these were fixation baseline scans and were positioned to bracket the six scans collected during encoding of a list of words presented through speakers. Each word list included 24 words, with one word presented every 2.5 seconds. The word lists were patterned after the CVLT (i.e., in a manner that allowed for assessment of the use of mnemonic organizational strategies). 30 Specifically, participants listened to word lists presented in three different conditions: a “directed” condition where participants were informed that the ensuing word list contained a categorical structure that should be used to enhance recall, a “spontaneous” condition where participants were presented with a word list that also contained a categorical structure of which the participants were not informed, and an “unrelated” condition in which the words had no obvious semantic or categorical relationship. For the directed and spontaneous conditions, the 24 words included four categories of six words each. Words from the same category were never presented in tandem. For the unrelated condition, all 24 words were from different categories. Each of these conditions included two trials, with the same words for each trial. The first spontaneous trial always preceded the first directed trial so that a participant’s first opportunity to initiate a semantic organizational strategy was evaluated while the participant was naive to the semantic structure inherent in the spontaneous and directed conditions. To control for order effects, presentation of the three conditions was counterbalanced across participants. As will be further detailed below, recall was assessed immediately following each of these encoding sessions.
Procedures and Formulas for Behavioral Measures of Memory and Strategy
Mnemonic performance was assessed through measures of free recall, cued recall, and recognition discriminability. Free recall, words recalled by each subject without provision of categorical clues from the investigator, was evaluated after each encoding condition (i.e., spontaneous, directed, and unrelated conditions). Free recall was calculated as the total number of words recalled minus intrusions and perseverations. Cued recall, words recalled in response to categorical prompting, was evaluated after the conditions that involved categories (i.e., the spontaneous and directed conditions). Cued recall was calculated with the same formula as free recall. For both free and cued recall, records of each subject’s responses were maintained by means of transcribing verbatim responses upon prompts appropriate for each particular condition. For example, for the spontaneous condition, participants were requested to “Repeat all the words you can remember from the list you just heard.” For the directed condition, participants were requested to recall words corresponding to a particular category. For example, if the category of interest happened to be clothes, participants were requested to “Please repeat all the words you can remember that were clothes.” This was done for each respective category in a serial manner. Recognition discriminability, an index used to correct for guessing, required yes-no responses upon verbal presentation of 48 words that included 24 correct targets and 24 distracters. It was administered after free and cued recall testing. The experimenter recorded the subject’s yes/no responses while reading from a list of 48 preprinted words. Recognition discriminability was calculated as: [1 − {false positives + false negatives)/48}] × 100.
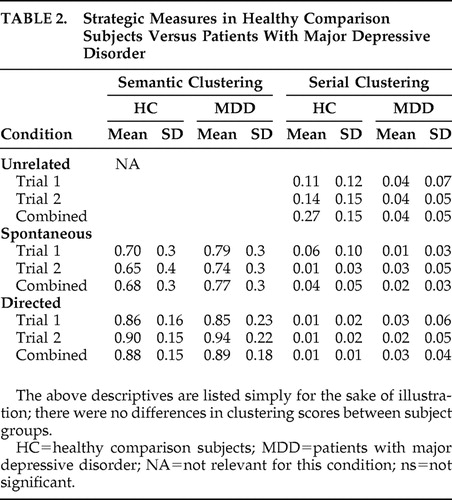
Strategic aspects of memory were evaluated through measures of semantic and serial clustering. Semantic clustering, the consecutive recall of 2 words from the same category, involved determining the total semantic clusters as a proportion of total possible semantic clusters. It was calculated as: [clusters/(words recalled − categories recalled)]. Semantic clustering scores were used as an index of a subject’s ability to employ semantic organizational strategies. In addition, subjects’ performance during the first spontaneous trial of free recall was used as a measure of semantic strategy initiation (i.e., a subject’s ability to realize that the word list contained a categorical structure and to further realize that recall might be enhanced by semantically clustering words). Serial clustering, the recall of two words in the same order as presented by the investigator, was calculated as a proportion of total possible clusters: [clusters/(words recalled − 1)]. 11 , 31 This was done separately for each trial, so participants had separate semantic and serial clustering scores for each trial of each condition (except for the unrelated condition, which did not offer the potential for semantic clustering).
Potential differences in performance for these indices were assessed by means of repeated measures analysis of variance (ANOVA) (trial × condition) and mixed model ANOVA (trial × condition × group). The effects of depression severity and IQ on memory performance were each evaluated by means of covariate analysis conducted as part of the ANOVA. Finally, memory performance was regressed against depression severity scores (i.e., BDI scores).
PET Scanning
All scanning occurred by means of the Scanditronix PC4096 scanner located at the Massachusetts General Hospital Department of Nuclear Medicine. The slice geometry consisted of contiguous slices with a center-to-center distance of 6.5 mm (axial field =97.5) and an in-plane resolution of 6.0 mm FWHM (full width half maximum). Scanning occurred during encoding of word lists while participants visually fixated on a black crosshair centered on a computer screen located approximately two feet from their head. Head position was maintained by means of an individually molded thermoplastic face mask and 15 O-labeled CO 2 was delivered by means of nasal cannulae and face mask.
PET images were reconstructed using a conventional convolution-backprojection algorithm, correcting for photon absorption, scatter, and dead time effects. The Hanning-weighted reconstruction filter was set to yield 8.0 mm in-plane spatial resolution FWHM. All scans were normalized to a regional cerebral blood flow of 50 ml/min per 100 g.
Image Analysis
Statistical analyses of the brain scans were conducted in accord with the theory of statistical parametric mapping (SPM). 32 Analysis followed standard realignment, normalization, and smoothing protocol included in the SPM2 software package program (Wellcome Department of Cognitive Neurology, London). Smoothing was processed at 20 mm in the x and y axes and at 15 mm in the z axis (FWHM). Post-processing analysis included entering the two scans for each condition as replicates. All statistical parametric maps were primarily thresholded at uncorrected p<0.001, which included a requirement for 5 contiguous voxels. Thresholding at the 0.005 level was also done to survey for regions of a priori interest not apparent at the p=0.001 level.
Analyses for a Priori Hypotheses
Contrasts were approached as empirical graded contrasts and as traditional subtraction contrasts. In terms of empirical graded contrasts, both subject groups were observed to perform better for the directed (D) condition than the spontaneous (S) condition, which in turn showed better performance than the unrelated (U) conditions (i.e., D>S>U). Thus, a corresponding empirically driven graded contrast was conducted for each group (i.e., D>S>U). This contrast was felt to reflect strategic processing in a general sense—a simultaneous evaluation of several cognitive processes related to encoding (initiation, semantic and serial clustering, idiosyncratic strategies, etc.).
Initiation and semantic organization were also evaluated by means of traditional subtraction contrasts. Specifically, a subtraction of S−D was conducted in order to evaluate the neural correlates of strategy initiation (i.e., the semantic organization resulting from a subject’s intrinsic realization that words from the list could be semantically clustered). From a conceptual perspective, the S−D contrast involved subtracting a word list condition where participants were explicitly provided with the organizational clustering strategy (D), from a naive condition (S) where participants were in the situation of potentially identifying the categorical nature of the list on their own and subsequently initiating the appropriate clustering strategy. Thus, subtracting a condition involving organization without initiation (D) from organization with initiation (S) allowed for isolation of the neural correlates of strategy initiation.
A traditional subtraction contrast of directed minus unrelated (D−U) was conducted to evaluate the neural correlates of semantic organization. That is, since the directed condition involved an effort to organize words without being forced to initiate an organizational strategy, and the unrelated condition involved a similar word list but did not involve organization nor strategy initiation, subtraction of the U from the D condition allowed for identification of the brain regions mediating semantic organization.
For the subtraction contrasts, the comparison of healthy comparison subjects minus patients with major depressive disorder was performed to identify regions of greater activation for initiation and organization in healthy comparison subjects relative to patients with major depressive disorder. The contrast of patients with major depressive disorder minus healthy comparison subjects was conducted to identify potential compensatory networks for these cognitive processes in depressed patients.
Post Hoc Imaging Analysis
To complement the aforementioned S−D contrast oriented toward identification of the neural correlates underlying semantic organization, we also conducted a subtraction of S1 from S2. That is, because the participants were truly naive to only the first spontaneous condition (S1), subjects presumably had the capacity to begin organizational processing in the second spontaneous trial (S2) (and possibly might have even begun this at the end of the first trial). Since strategic initiation would be expected to occur in both runs of the spontaneous condition (i.e., S1 and S2) and organizational processing would certainly be much more apparent by the second spontaneous condition, an S2−S1 contrast should identify the neural correlates of organization.
RESULTS
Participants
Patients with major depressive disorder had an average age of 36 years old (range=22–53) and an average IQ of 107 (range=101–118). Healthy comparison subjects had an average age of 34 years old (range=25–49) and an average IQ of 122 (range=111–132). The average total number of years of schooling for the depressed cohort was high school +3 years. The average total number of years of schooling for the healthy comparison subjects was high school +4 years. One of the healthy comparison subjects was enrolled in graduate school (philosophy student) and one of the depressed patients was in graduate school (biology student). The oldest patient with major depressive disorder also had the lowest IQ; she reported that problems with memory were most noticeable when her depressive symptoms were apparent. BDI scores revealed an average of 1.6 and 23 in healthy comparison subjects and patients with major depressive disorder, respectively. The two groups did not differ with respect to age (p>0.05) but did differ with respect to IQ and BDI scores (p<0.05 for both).
Neuropsychological Measures: Recall and Organizational Strategies
Healthy Comparison Subjects
Repeated measures ANOVA was conducted across both trials for each of the three basic mnemonic domains evaluated (i.e., free recall, cued recall, and recognition discriminability) ( Table 1 ), and for the two measures of mnemonic strategy (i.e., serial and semantic clustering) ( Table 2 ). In terms of free recall, there was a trend but not a significant effect of condition (i.e., D versus S versus U) (p=0.062). Neither cued recall nor recognition discriminability showed an effect for condition (p=1.0, p=0.53, respectively). Similarly, there was no effect for condition by trial interaction (p=0.33). In terms of strategic measures ( Table 2 ), semantic clustering did not show an effect for condition (p=0.147) or trial (p=0.925) but did show an effect for condition x trial interaction (p=0.047). Follow-up t test analysis for this interaction revealed that spontaneous trial 1 (T1) did not differ from spontaneous trial 2 (T2) (p=0.33), whereas directed T1 did differ from directed T2 (p=0.003). In contrast to free recall, serial clustering did show an effect for condition (p=0.04). Follow-up t test analysis revealed that the directed and unrelated conditions were different (p=0.020), as were the spontaneous and unrelated conditions (p=0.041). Serial clustering did not show an effect for condition by trial interaction (p=0.25).
 |
 |
Patients With Major Depressive Disorder
In terms of free recall, condition, trial, and condition by trial interaction, all showed significant effect (p=0.001, 0.002, 0.041, respectively) ( Table 1 and Table 2 ). Follow-up t test analysis revealed a significant difference between spontaneous and unrelated conditions (p=0.013) and the unrelated and directed conditions (p=0.01) but not the spontaneous and directed conditions (p>0.05). Evaluation of each condition for effect of trial showed a difference between T1 and T2 for the directed and unrelated conditions (p=0.001, p=0.018, respectively) but did not show an effect between the T1 and T2 trials of the spontaneous conditions (p=0.26). Covariate analysis employing BDI scores did not show any effect for the severity of depression (p=0.47). In contrast to free recall, neither cued recall (p=0.19) nor recognition discriminability (p=0.50) showed an effect for condition. In terms of strategic measures, semantic clustering did not show an effect for condition (p=0.31), nor condition × trial interaction (p=0.36). Serial clustering did not show any effect for condition (p=0.6), nor for trial by condition interaction (p=0.74). Regression of memory performance scores against BDI scores was not significant (p>0.05 in each case).
Healthy Comparison Subjects Versus Patients With Major Depressive Disorder
While performance differences were apparent between groups ( Table 1 ), there were no differences for strategic processes between groups ( Table 2 ). Reference to the summary of free recall scores in Table 1 reveals that comparison of healthy comparison subjects to patients with major depressive disorder did not show an effect for condition × group interaction (p=0.73) but did show significant effect for group × condition × trial interaction (p=0.024). Specifically, T1 and T2 of the directed condition both differed across groups (p=0.028, p=0.032, respectively), as did T2 of the spontaneous condition (p=0.026) and T2 of the unrelated condition (p=0.014). When considering the effect of IQ, covariate analysis revealed that all of these differences lost significance ( Table 1 ). In contrast to free recall, cued recall and recognition discrimination were not evaluated after each trial, thus these do not have condition × group × interaction analyses. Cued recall did not show an effect for condition by group interaction (p=0.30). Recognition discriminability did not show any difference between groups for the spontaneous and unrelated conditions (p=0.06, p=0.08, respectively), but did for the directed condition (p=0.008), an effect that was lost upon covarying for IQ.
In terms of strategic measures, semantic clustering showed an effect for condition × trial interaction (p=0.05), but no effect for group × condition × trial interaction (p=0.73). Serial clustering showed an effect for condition (p=0.02), but none for condition × group effect (p=0.08), nor for condition × group × trial interaction (p=0.21).
Imaging Results
Imaging results are summarized in Table 3 , stratified by z score. A priori regions of interest were tabulated if they had a p value equal to or less than 0.005. In addition, any findings with a p value less than or equal to 0.001 were tabulated. The following text highlights findings of particular interest.
 |
Graded Contrasts
Comparison of the unrelated, spontaneous, and directed conditions, stratified in accord with behavioral performance (i.e., D>S>U), revealed several sites of activation in depressed patients, but not healthy comparison subjects. For example, at the p≤0.001 level of significance, there were 10 sites for depressed patients versus only one for healthy comparison subjects. In regard to a priori regions of interest, patients with major depressive disorder showed activation of the left orbitofrontal cortex, right anterior cingulate cortex, and both hippocampi at the p<0.001 level. Of these, the right anterior cingulate cortex was the region showing the greatest z score (z=3.57) and is depicted in Figure 1 . Healthy participants showed activation of the left caudal ventrolateral prefrontal cortex (p=0.001), left rostral ventrolateral prefrontal cortex (p=0.002), and the right ventrolateral prefrontal cortex (p=0.003). Of these, the left caudal ventrolateral prefrontal cortex region showed the greatest z score (z=3.04) ( Figure 2 ).
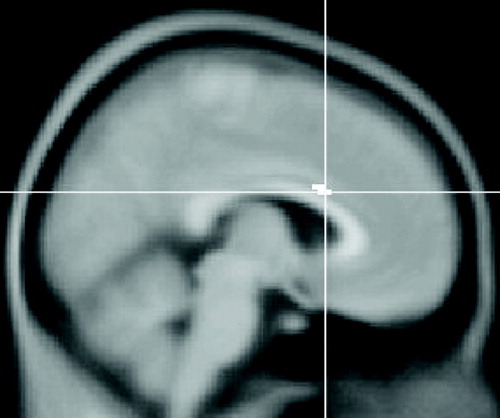
This image is a sagittal section. Voxels coded in white identify the right anterior cingulate cortex (MNI coordinates 6, 18, 24) as the neuroanatomical site of greatest z score during encoding.

This image is a sagittal section. Voxels coded in white identify the caudal left ventrolateral prefrontal cortex (MNI coordinates −38, 20, 4) as the neuroanatomical site of greatest z score during encoding.
Subtraction Analyses
The spontaneuous−directed contrast showed greater activation of the right anterior cingulate cortex, left ventrolateral prefrontal cortex, and the right hippocampus, all at p<0.001, as well as the bilateral orbitofrontal cortex (p=0.002), in healthy comparison subjects relative to patients with major depressive disorder. Patients with major depressive disorder showed greater recruitment of the right ventrolateral prefrontal cortex relative to healthy comparison subjects.
The directed−unrelated contrast showed greater activation of the left ventrolateral prefrontal cortex in healthy comparison subjects relative to patients with major depressive disorder (p<0.001). Patients with major depressive disorder showed recruitment of the right anterior cingulate cortex relative to healthy comparison subjects (p<0.001).
Post Hoc Imaging Results
For healthy comparison subjects, the S2−S1 contrast (i.e., the second spontaneous trial minus the first spontaneous trial) showed activations of the left orbitofrontal cortex, ventrolateral prefrontal cortex, and middle and superior temporal gyri (p<0.001). For patients with major depressive disorder, the S2−S1 contrast revealed increased activation of the left orbitofrontal cortex, pregenual cingulate, frontal pole, and middle frontal gyrus, as well as the right ventrolateral prefrontal cortex and frontal and occipital poles (p<0.001 in all cases) ( Table 3 ).
DISCUSSION
The results from this study can be summarized in terms of behavioral/mnemonic findings and neuroimaging findings. In regard to memory performance, of the general domains of memory evaluated (i.e., free recall, cued recall, and recognition discriminability), only free recall showed a difference between healthy comparison subjects and patients with major depressive disorder. This difference, however, was lost upon covariate analysis for IQ. That is, the observed performance differences may be secondary to differences in IQ, as opposed to the depressed state. Evaluation of performance on measures of mnemonic strategy (i.e., serial and semantic clustering) did not reveal any differences between our subject groups.
Our neuropsychological findings are similar to those of other investigators; for example, neither Bremner et al., 9 Wang et al., 16 nor Basso and Bornstein 33 showed differences in memory between healthy comparison subjects and patients with major depressive disorder. The comparable performance between these groups may, however, need to be interpreted in the context of the severity of the patients’ depression. For example, our depressed patients had a mean BDI score of 23, which is consistent with mild depression. Basso and Bornstein 33 have shown that patients with mild major depressive disorder have comparable memory scores to healthy comparison subjects (i.e., only patients with more severe major depressive disorder have memory deficits relative to healthy comparison subjects). 4 , 34
Conversely, it can be argued that neural correlates are a more sensitive index of brain function than are behavioral or neuropsychological tests. For example, a sample size of eight subjects probably does not provide adequate power to detect differences in neuropsychological performance, but PET imaging can identify putative sites of neural function with this sample size. 35 In this regard, our neuroimaging results had significant findings in terms of both the empirical graded contrasts and the traditional subtraction contrasts. The graded contrasts showed 10 sites of significant activation in patients with major depressive disorder and only one site in healthy comparison subjects. The sites affected in our depressed patients provide evidence for involvement of frontal-subcortical circuits (e.g., the left ventrolateral prefrontal cortex and caudate) and prefrontal-hippocampal circuitry (e.g., the right anterior cingulate cortex and hippocampus) 1 and provide additional support for the notion that encoding in depressed patients may require more extensive network activation than is necessary in healthy persons. 10
The neuroimaging component of our study has replicated findings from other studies that also evaluated the neural correlates of episodic memory in patients with major depressive disorder by providing further evidence for aberrant function of the hippocampus and anterior cingulate cortex. 9 , 10 The novel contributions of our study involved an assessment of the neural correlates of strategic processes employed during encoding of episodic memory. In this regard, during strategy initiation (i.e., S−D), healthy subjects showed increased activation of the right orbitofrontal cortex, right anterior cingulate cortex, right hippocampus, and the left ventrolateral prefrontal cortex relative to depressed patients, whereas the depressed patients showed increased activation of the right ventrolateral prefrontal cortex relative to healthy comparison subjects. The network recruited by the healthy comparison subjects includes all the a priori sites predicted for initiation (i.e., the orbitofrontal cortex and anterior cingulate cortex; the hippocampus [recruited during novel stimuli]; 23 , 36 and the left ventrolateral prefrontal cortex [recruited during organization]), whereas the right ventrolateral prefrontal cortex (as recruited by the depressed cohort) has not generally been shown to be associated with initiating strategic processes. Thus, our finding of right ventrolateral prefrontal cortex activation in depressed patients may identify an aberrant site of compensatory function in patients with depression. Similar findings have been shown by other investigators (e.g., Assaf et al. 37 have suggested that a right-sided network is preferentially activated in patients with schizophrenia—and possibly psychiatric disease in general). Increased activation of the right inferior frontal gyrus and middle frontal gyrus was also observed in a cohort of patients with major depression in an episodic memory study by Gron and coworkers, 38 which evaluated memory for geometric figures.
Although our S−D contrast was intended to isolate neural correlates associated with initiation, it is notable that the second trial of the spontaneous condition probably involved organization. For example, as participants processed the first trial of the spontaneous condition, they could have realized that the spontaneous condition included a categorical structure; thus, the potential for semantic organization may have arisen during the first trial and could have been in mind at the start of the second spontaneous trial. Evidence for this line of reasoning is apparent upon evaluation of the S2−S1 contrast within both subject groups. Because the participants are no longer naive to the potential for semantic organization by the time the second spontaneous trial is presented, one would predict that comparison of the S1 and S2 trials should reveal greater activation of the left ventrolateral prefrontal cortex (or dorsolateral prefrontal cortex) in S2 relative to S1. And, indeed, this is apparent for both subject groups (see Post Hoc Results: the left ventrolateral prefrontal cortex in healthy comparison subjects and the left middle frontal gyrus and right ventrolateral prefrontal cortex in depressed patients).
Complementing our contrast for strategic initiation, our contrast for semantic organization revealed that patients with major depressive disorder showed reduced activation of the left ventrolateral prefrontal cortex and increased activation of the right anterior cingulate cortex relative to healthy comparison subjects. Reduced activation of the ventrolateral prefrontal cortex is consistent with our a priori hypothesis, whereas increased activation of the anterior cingulate cortex is opposite from what we predicted. Similar results (for the anterior cingulate cortex) have been observed by others for working memory, 39 , 40 for encoding of visual episodic memory, 38 and for encoding of episodic sad stimuli, as observed by Fahim and coworkers, 41 who suggested that increased activation of the anterior cingulate cortex may reflect increased effort on the part of depressed patients.
Of special note, for both subtraction contrasts, the depressed patients showed a pattern of reduced activation of regions typically expected to mediate certain processes (e.g., the left ventrolateral prefrontal cortex and semantic organization) and a greater activation of regions less typically recruited during these processes. Specifically, the right ventrolateral prefrontal cortex has been repeatedly implicated in semantic encoding, but it may not have as primary a role as does the left ventrolateral prefrontal cortex for encoding. 5 This pattern of right-sided preference in depressed patients may suggest that network function in patients with major depressive disorder is characterized by reduced activity in what might be considered primary networks (i.e., semantic encoding typically relies on the left ventrolateral prefrontal cortex) and a compensatory increase in what might be considered secondary networks (e.g., use of the right ventrolateral prefrontal cortex for semantic encoding). 42
The foregoing combination of aberrant network activity in depressed patients and lack of a difference in behavioral performance is consistent with the findings of Bremner et al., 9 who have shown that even when depressed patients perform comparably to healthy comparison subjects, they still have reduced activation of the networks mediating the task. Studies by Basso and Bornstein 33 and by McCall and Dunn 43 have shown that neuropsychological impairments do exist in patients with major depressive disorder, but these impairments only present after several depressive episodes—or in the severely depressed. In synthesis of these findings, we postulate that the neural network aberrations we have observed may be precursors of the neuropsychological impairments. That is, as a depressive episode worsens, networks that were beginning to show dysfunction during the initial or mild stages of the episode may suffer further functional deterioration to then eventually manifest with clinical symptomatology.
The limitations of our study deserve discussion. Foremost, our subject groups were not matched for IQ. As discussed above, this limitation could account for the behavioral differences, but it could also account for the network activation differences. For example, it has been shown that IQ differences can be associated with differences in both network activation and neural structure. 44 Moreover, in women, IQ has been shown to correlate with a gray matter volume in Broca’s region, an area central to verbal memory function. 45 , 46 The CVLT is obviously a verbal memory task, and our higher IQ group (i.e., our healthy comparison subjects) may have activated the left ventrolateral prefrontal cortex to a greater degree simply because of the structure, activation, and connectivity profile consistent with a higher IQ and not because of the absence of depressive symptoms. 47 However, in refutation of this explanation, Halari and coworkers 48 have evaluated cognitive processes such as attention and error detection in pediatric healthy comparison subjects matched for IQ with a depressed pediatric cohort and have shown aberrant activation of the lateral prefrontal cortex and anterior cingulate cortex in the depressive cohort (i.e., findings similar to ours). Combining the results from Halari et al. 48 with those from Assaf et al. 37 (right-sided findings in psychiatric populations) and Gron et al. 38 (increased activation of the anterior cingulate during encoding), it would appear unlikely that IQ differences alone account for the network activations found in our study.
A second shortcoming of our paradigm was the lack of synchronization of subjects by phase of the menstrual cycle. Verbal memory has been shown to be enhanced by E2, and E2 has been shown to drive activity across prefrontal-hippocampal systems. 49 , 50 Because women with depression can have reduced E2 levels relative to healthy comparison subjects, 51 synchronization of subjects by phase of menstrual cycle (or perhaps correlation of blood E2 levels across subjects) might have resulted in producing subject groups with different E2 levels (e.g., lower levels in subjects with major depressive disorder) and thus may have concurrently resulted in revealing performance differences between groups and/or may have informed the network activations. A third potential limitation of our study was our use of subtraction analyses to “isolate” components of cognitive processes. It should be appreciated that while this strategy may provide rudimentary localizing information aimed at a dimension of a cognitive process, 52 , 53 cognitive processes are highly interwoven phenomenon and subtraction analyses probably do not address the complexity of the issue. 54 In this regard, however, each of our subtraction analyses did identify regions of putative significance for the cognitive dimension of interest. Finally, our study evaluated only eight patients with major depressive disorder and eight healthy comparison subjects. This sample size both limited the generalizability of our findings and made it unrealistic to correct for multiple comparisons.
In conclusion, our study has provided evidence for aberrant function of networks known to underlie organization and semantic processing during a task designed to interrogate the functional integrity of the corresponding prefrontal networks. Thus, our functional neuroimaging findings were consistent with our a priori hypotheses. Similarly, our behavioral data were consistent with our a priori hypothesis of no differences in memory between healthy comparison subjects and mildly depressed patients. 33 Given these findings, future neuroimaging studies evaluating neural network function in mildly depressed patients versus severely depressed patients would be of great interest. Indeed, such a comparison may show that the degree to which network activations are attenuated may correlate with the severity of a depressive episode and with clinical manifestation of neuropsychological impairments. Moreover, additional insight into the pathophysiology of major depressive disorder may emerge as cognitive and affective imaging studies not only addresses network activations but also evaluate aberrant network connectivity and the neurobiological variables (e.g., genetic predisposition, hormonal effects, etc.) that may contribute to these aberrations. 49 , 55 , 56
1. Ottowitz WE, Dougherty DD, Savage C: The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry 2002; 10:86–99Google Scholar
2. Caine ED: Pseudodementia: current concepts and future directions. Arch Gen Psychiatry 1981; 38:1359–1364Google Scholar
3. Kizilbash AH, Vanderploeg RD, Curtiss G: The effects of depression and anxiety on memory performance. Arch Clin Neuropsychol 2002; 1:57–67Google Scholar
4. Deuschle M, Kniest A, Niemann H, et al: Impaired declarative memory in depressed patients is slow to recover: clinical experience. Pharmacopsychiatry 2004; 37:147–151Google Scholar
5. Tulving E, Kapur S, Craik FIM, et al: Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA 1994; 91:2016–2202Google Scholar
6. Hamilton JM, Salmon DP, Galasko D, et al: A comparison of episodic memory deficits in neuropathologically-confirmed dementia with Lewy bodies and Alzheimer’s disease. J Int Neuropsychol Soc 2004; 10:689–697Google Scholar
7. Baldo JV, Delis D, Kramer J: Memory performance on the California Verbal Learning Test-II: findings from patients with focal frontal lesions. J Int Neuropsychol Soc 2002; 8:539–546Google Scholar
8. Otto MW, Bruder GE, Fava M, et al: Norms for depressed patients for the California Verbal Learning Test: associations with depression severity and self-report of cognitive difficulties. Arch Clin Neuropsychol 1994; 9:81–88Google Scholar
9. Bremner JD, Vythilingam M, Vermetten E, et al: Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 2004; 161:637–645Google Scholar
10. De Asis JM, Stern E, Alexopoulous GS, et al: Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry 2001; 158:1321–1323Google Scholar
11. Savage C, Deckersbach T, Heckers S, et al: Prefrontal regions supporting spontaneous and directed application of verbal learning strategies. Brain 2001; 124:219–231Google Scholar
12. Simmons A, Miller D, Feinstein JS, et al: Left inferior prefrontal cortex activation during a semantic decision-making task predicts the degree of semantic organization. Neuroimage 2005; 28:30–38Google Scholar
13. Elliot R, Baker SC, Rogers RD, et al: Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 1997; 27:931–942Google Scholar
14. Pillow DR, Dougherty RW: Dysphoria and the failure to perceive and use discounting information: implications for internalizing negative feedback. Percept Mot Skills 1996; 83:107–113Google Scholar
15. Lafont V, Medecin I, Robert PH, et al: Initiation and supervisory processes in schizophrenia and depression. Schizophr Res 1998; 34:49–57Google Scholar
16. Wang CE, Halvorsen M, Sundet K, et al: Verbal memory performance of mildly to moderately depressed outpatient younger adults. J Affect Disord 2006; 92:283–286Google Scholar
17. Posner MI, Petersen SE, Fox PT, et al: Localization of cognitive operations in the human brain. Science 1998; 240:1627–1631Google Scholar
18. Kaneda M, Osaka N: Role of the anterior cingulate cortex during semantic coding in verbal working memory. Neurosci Lett 2008; 436:57–61Google Scholar
19. Petrides M: Lateral prefrontal cortex: architectonic and functional organization. Phil Trans Roy Soc London B 2005; 360:781–795Google Scholar
20. Demb JB, Desmond JE, Wagner AD, et al: Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci 1995; 15:5870–5878Google Scholar
21. Basho S, Palmer ED, Rubio MA, et al: Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia 2007; 45:1697–1706Google Scholar
22. Cabeza R, Nyberg L: Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cog Neurosci 2000; 12:1–47Google Scholar
23. Kesner RP, Lee I, Gilbert P, et al: A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 2004; 15:333–351Google Scholar
24. Lavretsky H, Kurbanyan K, Ballmaier M, et al: Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry 2004; 12:653–657Google Scholar
25. Keightley ML, Seminowicz DA, Bagby RM, et al: Personality influences limbic-cortical interactions during sad mood induction. Neuroimage 2003; 20:2031–2039Google Scholar
26. Marcus SM, Young EA, Kerber KB, et al: Gender differences in depression: findings from the STAR*D study. J Affect Disord 2005; 87:141–150Google Scholar
27. Goodwin RD, Gotlib IH: Gender differences in depression: the role of personality factors. Psychiatry Res 2004; 126:135–142Google Scholar
28. First MB, Spitzer RL, Gibbons M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID), Version 2.0. New York, Biometrics Research Department, New York Psychiatric Institute, 1995Google Scholar
29. Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory—II. San Antonio, Tex, Psychological Corp, 1996Google Scholar
30. Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test: Manual. San Antonio, Tex, Psychological Corp, 1987Google Scholar
31. Stricker JL, Brown GG, Wixted J, et al: New semantic and serial clustering indices for the California Verbal Learning Test—2nd ed: background, rationale, and formulae. J Int Neuropsychol Soc 2002; 8:425–435Google Scholar
32. Friston KJ, Holmes AP, Worsley K, et al: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1994; 2:189–210Google Scholar
33. Basso MR, Bornstein RA: Relative memory deficits in recurrent versus first episode major depression on a word learning task. Neuropsychology 1999; 13:557–563Google Scholar
34. Porter RJ, Gallagher P, Thompson JM, et al: Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 2003; 182:214–220Google Scholar
35. Nobre AC, Sebestyen GN, Gitelman DR, et al: Functional localization of the system for visuospatial attention using positron emission tomography. Brain 1997; 120:515–533Google Scholar
36. Grunwald T, Kurthen M: Novelty detection and encoding for declarative memory within the human hippocampus. Clin EEG Neurosci 2006; 37:309–314Google Scholar
37. Assaf M, Rivkin PR, Kuzu CH, et al: Abnormal object recall and anterior cingulate over activation correlate with formal thought disorder in schizophrenia. Biological Psychiatry 2006; 59:452–459Google Scholar
38. Gron G, Bittner D, Schmitz B, et al: Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol 2002; 51:491–498Google Scholar
39. Matsuo K, Glahn DC, Peluso MA, et al: Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 2007; 12:158–166Google Scholar
40. Rose EJ, Simonotto E, Ebmeier KP: Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage 2006; 29:203–215Google Scholar
41. Fahim C, Stip E, Mancini-Marie A, et al: Abnormal prefrontal and anterior cingulate activation in major depressive disorder during episodic memory encoding of sad stimuli. Brain Cogn 2004; 54:161–163Google Scholar
42. Lee AC, Robbins TW, Pickard JD, et al: Asymmetric frontal activation during episodic memory: the effects of stimulus type on encoding and retrieval. Neuropsychologia 2000; 38:677–692Google Scholar
43. McCall WV, Dunn AG: Cognitive deficits are associated with functional impairment in severely depressed patients. Psychiatry Res 2003; 121:179–184Google Scholar
44. Choi YY, Shamosh NA, Cho SH: Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neuroscience 2008; 41:10323–10329Google Scholar
45. Haier RJ, Jung RE, Yeo RA, et al: The neuroanatomy of general intelligence: sex matters. Neuroimage 2005; 25:320–327Google Scholar
46. Schmithorst VJ, Holland SK: Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage 2007; 35:406–419Google Scholar
47. Heun R, Klose U, Jessen F, et al: Functional MRI of cerebral activation during encoding and retrieval of words. Hum Brain Mapp 1999; 8:157–169Google Scholar
48. Halari R, Simic M, Pariante CM, et al: Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol Psychiatry 2009; 50:307–316Google Scholar
49. Ottowitz WE, Siedlecki K, Lindquist MA, et al: Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion. Psychoneuroendocrinology 2008; 33:1419–1425Google Scholar
50. Zec RF, Trivedi MA: The effects of estrogen replacement therapy on neuropsychological functioning in postmenopausal women with and without dementia: a critical and theoretical review. Neuropsychol Rev 2002; 12:65–109Google Scholar
51. Young EA, Midgley AR, Carlson NE: Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry 2005; 57:1157–1162Google Scholar
52. Lie CH, Specht K, Marshall JC, et al: Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 2006; 30:1038–1049Google Scholar
53. Kim JJ, Andreasen NC, O’Leary DS, et al: Direct comparison of the neural substrates of recognition memory for words and faces. Brain 1999; 122:1069–1083Google Scholar
54. Friston KJ, Price CJ, Fletcher P, et al: The trouble with cognitive subtraction. Neuroimage 1996; 4:97–104Google Scholar
55. Wolfensberger SP, Veltman DJ, Hoogendijk WJ, et al: The neural correlates of verbal encoding and retrieval in monozygotic twins at low or high risk for depression and anxiety. Biol Psychol 2008; 79:80–90Google Scholar
56. Vasic N, Walter H, Sambataro F, et al: Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychol Med 2008; 10:1–11Google Scholar



