Possible Polyneuritis Cranialis in a Psychotic Patient: Diagnostic and Therapeutic Dilemmas
To the Editor: The case reported here refers to a 42-year-old single woman with a previous history of bipolar I disorder (with onset at the age of 28) with psychotic features and hypothyroidism. Until the index hospitalization, she suffered from several relapses and consequent hospitalizations. During the last year, she was receiving 100 mg/day topiramate and 0.1 mg/day thyrohormone. The index hospitalization occurred when she presented to the emergency unit with disorganized and agitated behavior, plus noncongruent psychotic features.
On admission, she received haloperidol 10 mg intramuscularly (i.m.), diazepam 10 mg i.m., and biperiden 10 mg i.m. Her treatment during her hospitalization (which lasted for 57 days) is shown in detail in Figure 1, except for benzodiazepines, long-acting risperidone i.m., valproate, and thyrohormone. The fluctuation of the clinical picture is also shown in the same figure. On Day 7, she manifested hypersalivation and peripheral muscular rigidity. There was a disagreement among therapists concerning the etiology of the rigidity, because of the lack of cogwheel and other extrapyramidal signs and symptoms (EPS). However, she was treated with biperiden. The disappearance of rigidity does not seem to temporally relate to the anticholinergic treatment. Her treatment included high dosages of olanzapine and haloperidol later on, but the symptoms persisted. On Day 18, she had a 38.5°C fever, treated with paracetamol and antibiotics. Clinical and laboratory examinations were normal. In order to control her disorganized behavior, high dosages of olanzapine were used, on a step-by-step basis, during Days 17–23, and the patient seemed to respond at dosages close to 100 mg daily. However, the core symptoms persisted. On Day 21, she manifested severe hypersalivation, hoarseness of voice, but no peripheral muscular rigidity. On Day 25, her treatment changed, and the new one was based on haloperidol 30 mg daily and valproate 1,500 mg daily.
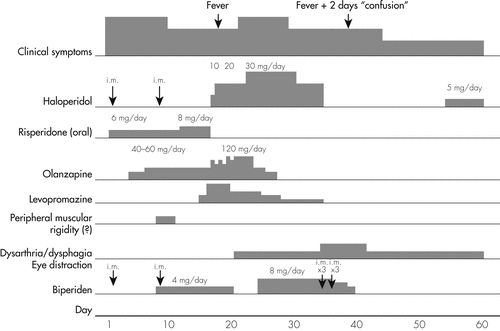
On Day 35, she manifested severe dysphagia, dysarthria, and hypersalivation in the absence of peripheral muscular dysfunction. The phenomena were considered to be extrapyramidal in nature, and all antipsychotic treatment was stopped. Long-acting risperidone was stopped also. Brain magnetic resonance imaging (MRI) was normal. On Day 39, she suffered from a second fever episode (37.8°C), and during Days 39–41, she was lethargic. Biperiden was stopped. A significant decrease in thyroid-stimulating hormone (TSH) was found, whereas the thyroid ultrasound and the neck computerized tomography (CT) were normal. Thyrohormone was decreased to 0.075 mg/day. Her treatment was 15 mg/day diazepam and 1,000 mg/day valproate sodium for the following days. On Day 45, a new neurological examination revealed the presence of skew deviation1 and confirmed the presence of dysphagia and dysarthria. During the next 12 days, the patient began improving, but neither the psychiatric symptoms nor the dysarthria, the dysphagia, or the skew deviation remitted completely. She was released and put on outpatient follow-up. On release, because of the presence of a slight hyperthymia, 5 mg haloperidol was added, and she continued to also receive 50 mg long-acting risperidone.
The patient was hospitalized again 3 months later because of a relapse in her psychotic symptoms, which remitted within 48 hours after aggressive treatment with 1,800 mg quetiapine daily and 400 mg amisulpride daily. No EPS emerged. She manifested a complete recovery from her psychiatric symptoms.
She was followed up once to twice per month on an outpatient basis. No peripheral EPS were ever evident. Dysphagia, dysarthria, and skew deviation continued to improve slowly and disappeared after another 2.5 months; that is, 6 months after they first appeared.
The fact that none of the motor symptoms responded to anticholinergic treatment, that they persisted for more than 1 month after stopping the high dosages of haloperidol, and their independent fluctuation, argue against an extrapyramidal causation. The most probable cause could be multiple cranial neuritis (polyneuritis cranialis; PC), which is a rare disorder of multiple cranial nerve palsies without spinal cord involvement. It is thought to be an acute postinfective polyneuropathy or a variant of Guillain-Barré syndrome. Electrophysiological evidence of demyelination has been reported, but no radiological abnormalities of the affected cranial nerves has been noted.2–6 An additional feature, which was not assessed at that time, but only retrospectively, is a significant reduction in the mimic of face muscles, which can easily be considered as an extrapyramidal feature, but is also a common element of PC. Sialorrhea could also be considered in the same context.
1 : Eye movement disorders. Curr Opin Ophthalmol 1995; 6:27–33Crossref, Medline, Google Scholar
2 : Natural course of acute and chronic monophasic inflammatory demyelinating polyneuropathies (IDP): a retrospective analysis of 266 cases. Acta Neurol Scand 1992; 85:282–291Crossref, Medline, Google Scholar
3 : Landry-Guillain-Barré syndrome with cranial nerve involvement (polyneuritis cranialis). Harper Hosp Bull 1964; 22:59–62Medline, Google Scholar
4 : Multiple cranial neuritis associated with large granular lymphocytosis. Acta Haematol 1994; 92:157–159Crossref, Medline, Google Scholar
5 : Polyneuritis cranialis as the sole manifestation of the Guillain-Barré syndrome. Mo Med 1976; 73:227–229Medline, Google Scholar
6 : Polyneuritis cranialis: clinical and electrophysiological findings. J Neurol Neurosurg Psychiatry 1992; 55:398–400Crossref, Medline, Google Scholar



