Differences in Anxiety Among Patients With Early- Versus Late-Onset Alzheimer’s Disease
Abstract
The authors sought to evaluate the incidence and correlates of anxiety in early-onset Alzheimer’s disease (EOAD) versus the more typical late-onset AD (LOAD). A group of 23 EOAD and 22 LOAD patients were compared by the Neuropsychiatric Inventory Anxiety subscale. Demographic and disease-related relationships with anxiety were evaluated, as well as types of anxiety symptoms that were endorsed. EOAD patients had significantly more anxiety symptoms than LOAD patients. Among those with EOAD, anxiety was associated with male gender, higher Mini-Mental State Exam score, and separation from caregivers. Among LOAD patients, anxiety was associated with psychotic and activating psychiatric symptoms. These results have implications for the management and alleviation of anxiety in AD.
Anxiety is a significant complication of dementia, but it has not received as much attention as other psychiatric features, such as depression and psychotic symptoms.1,2 Anxiety is more common among patients with dementia than in the general population, and prevalence estimates for patients with dementia range from 5%−21% for anxiety disorders3–7 and from 8%−81% for anxiety symptoms.3,8–10 The large range in reported prevalence may be due to difficulty operationalizing anxiety separate from comorbid symptomatology such as agitation and irritability in dementia. For example, between 68% and 75% of individuals with dementia and Generalized Anxiety Disorders also meet criteria for Major Depressive Disorder.4,7
Anxiety may be particularly associated with Alzheimer’s disease (AD).6,8,11–14 There are greater levels of anxiety in AD than in non-cognitively impaired individuals.12,13 Similarly, in studies commenting on anxiety in AD patients, the cross-sectional variance has ranged from 25% to 75%.12,15–20 Anxiety in AD is associated with depression, tearfulness, irritability, overt aggression, and mania.3,21 Anxiety in AD has not shown a consistent association with sex, education, or ethnicity,14,22–26 but greater levels of anxiety occur among those with retained insight and in earlier, less profound stages of dementia.21,25–28
Another important factor affecting the incidence of anxiety in AD is the age at onset. The literature is increasingly demonstrating that there are significant clinical and neurobiological differences between early-onset Alzheimer’s disease (EOAD), with an onset before age 65, and the more-typical, late-onset form of the disorder (LOAD).29–33 Despite the fact that there are psychosocial and cognitive differences between EOAD and LOAD, few studies have examined differences in the incidence of anxiety on the basis of differences in age at onset of AD. Of these studies, the literature is inconclusive with regard to what groups are at greater risk for anxiety;34–36 some studies have found that anxiety is greater in older patients with AD,4,37 whereas others have found no relationship between age and anxiety in this disorder.24 In another investigation, using the Neuropsychiatric Inventory (NPI), researchers found anxiety to be more prevalent among patients with EOAD than LOAD.14 They also found that anxiety correlated with greater functional limitations.14 These investigations have not identified other variables that may explain or account for anxiety differences between patients with EOAD and LOAD.
The current study built on this previous work with the NPI, using a well-characterized cohort of patients with EOAD and with LOAD to further explore the potential correlates and underlying mechanisms for differentiating anxiety between these patient groups. This study hypothesized that patients with EOAD, as compared with those with LOAD, would have higher levels of anxiety, as this disease strikes at the height of their family and career responsibilities, resulting in a greater functional impact than in those with LOAD. This article aims to clarify the prevalence of anxiety among those with EOAD, as compared with LOAD, and further explores the factors that underlie anxiety in these patients.
Methods
Participants
A sample of 23 EOAD and 22 LOAD participants were recruited from the Departments of Neurology and Geriatric Psychiatry at the Veterans Affairs (VA) Greater Los Angeles Healthcare Center and the University of California, Los Angeles (UCLA) Geffen School of Medicine. This was a convenience sample, building upon a previous study investigating differences between EAOD and LOAD.38 Diagnosis of AD was determined by the following procedures: a baseline work-up including a clinical history, dementia rating scales, a neurological examination, opthamological examination, routine laboratory tests (ruling out reversible causes of dementia), neuropsychological testing, and clinical neuroimaging (MRI). A diagnostic consensus conference was held, including nurses, a neuropsychologist, and physicians who were not involved in the patient’s clinical care. The study included all patients meeting criteria for clinically-probable AD, based on the National Institute of Communicable Diseases and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA).39 We defined diagnosis group by “early-onset” versus “late-onset” as determined by age at onset; those with a disease onset before age 65 were deemed to be “early-onset,” and those with a disease onset subsequent to age 65 were deemed to be “late-onset.” Additional exclusion criteria were the presence of active or unstable medical problems, including significantly reduced renal, liver, or cardiac function; diabetes mellitus with blood glucose levels >180; uncontrolled hypertension (>180/100); history of previous head injury with loss of consciousness; systemic or other neurological condition that could account for dementia; history of a psychotic disorder unrelated to dementia; or a history of a psychoactive substance use disorder. All participants were community-dwelling, living with their caregivers, or on their own, at the start of the study. The study was reviewed and approved by the local Institutional Review Board (IRB), and study participants were enrolled according to IRB guidelines.
Procedures
Participants engaged in brief neurocognitive screening. Caregivers (study partners) who accompanied patients to appointment filled out questionnaires to provide information regarding their experiences/impressions of the patient.
Measures
Demographics
Both participants and study partners (caregivers) provided information such as age, education level, marital and living-situation status, and occupation. Disease-related information, including diagnosis and age at onset, was also provided by caregivers.
Global Cognitive Severity and Functional Status (ADLs/IADLs)
All participants completed the Mini-Mental State Exam (MMSE) as a measure of global cognitive impairment. The data were normed, adjusting for age and education level.40 In order to assess functional status, all participants completed the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory (ADCS-ADL);41 a 23-item inventory wherein the caregiver is asked to focus on the patient’s observed performance over the previous month.
Anxiety
The Neuropsychiatric Inventory (NPI) measures the frequency and severity of 12 psychiatric and behavioral symptoms/domains on the basis of caregiver report42 (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep disturbance, and appetite and eating disorder).43 For each domain, the NPI begins with a screening question (Yes or No, to indicate presence versus absence of this symptom), followed by 7–8 more specific follow-up questions to determine the nature of the overall symptom endorsed (again, in Yes/No format). For anxiety, the initial screening question asks: “Is the patient very nervous, worried, or frightened for no apparent reason? Does he/she seem very tense or fidgety? Is the patient afraid to be apart from you?; the specific follow-up questions are: worrying about planned events; feeling shaky; unable to relax or tense; shortness of breath or gasping; butterflies in stomach or racing/pounding heart; avoidance of certain situations; becoming nervous when separated from caregiver; and other symptoms of anxiety. The caregiver is asked to rate the overall frequency1–4 and severity1–3 of anxiety symptoms, thus providing an overall index of symptom severity. Test–retest reliabilities for this measure range from 0.64 to 0.71.42,44
Statistical Analysis
Descriptive statistics and frequencies for demographic and clinical characteristics were generated by diagnostic group for patient and caregiver. Differences in these characteristics between EOAD and LOAD were compared, using chi-square (χ2) or t-tests (or Wilcoxon rank tests) for categorical (dichotomous and ordinal) or continuous variables, respectively. Logistic-regression models were used to assess 1) whether the odds of experiencing anxiety were different between diagnostic groups (unadjusted analysis); 2) whether presence of anxiety was associated with each of the demographic variables within diagnosis group (stratified analysis); and 3) whether these associations differed by diagnostics group (a single modeling approach). When we investigated the relationship between each of the pre-selected demographic variables and anxiety within each diagnosis group (stratified analyses), we found the relationships between presence of anxiety and gender or MMSE differed by diagnostics group. Thus, we used a single modeling approach, including the main effects (group, gender, or MMSE) and a group × variable interaction (i.e., group × gender or MMSE) to test whether these associations were significantly different. Chi-square tests were conducted to assess the association between each of the specific types of anxiety symptoms and diagnosis group. Last, Spearman correlations (using ordinal measures of psychiatric symptom indices, frequency × severity) were conducted to examine relationship of anxiety scale scores to other measures on the NPI. Because of the moderate sample size and fact that results were to serve as preliminary data, we did not correct for multiple comparisons, in order to see whether there was any effect. All statistical analyses were carried out with SPSS 20.0 and SAS System for Windows (Version 9.2). The significance level was set at 0.05 for all analyses (i.e., p <0.05).
Results
Demographics of Patients and Caregivers
There were no significant group differences with regard to patients’ MMSE score, time since diagnosis, education (most participants across the samples had some college experience), gender, handedness, or family history of dementia (see Table 1), except ethnicity (χ2=3.76; p=0.049). Data for medications the patients were taking are also provided in Table 1, and there was only a significant association of medications for other general-medical conditions and diagnosis group (χ2=4.66; p=0.03). The association between anxiety and medication status was not significant (psychiatric medications: χ2=1.89; NS; acetylcholinesterase inhibitors: χ2=1.42; NS; general-medical condition medication: χ2=2.65; NS).
| Demographic Variables | Early-Onset Alzheimer’s Disease (N=23) | Late-Onset Alzheimer’s Disease (N=22) |
|---|---|---|
| Patient Information | ||
| Age, years*** | 57.68 (4.19) | 80.32 (5.89) |
| Gender, N (%) | ||
| Men; women | 16 (64%); 9 (36%) | 15 (68%); 7 (32%) |
| Education, years | 16 (64%); 9 (36%) | 15 (68%); 7 (32%) |
| Handedness, N (%) | ||
| Right; left; ambidextrous | 22 (88%); 1 (4%); 1 (4%) | 21 (95%); 1 (4.5%); 0 |
| Time since onset, years | 3.09 (1.55) | 3.91 (2.76) |
| Family history of dementia, N (%) | ||
| Yes; no; unsure | 8 (32%); 13 (52%); 4 (16%) | 11 (50%); 11 (50%); 0 (0%) |
| Mini-Mental State Exam | 3.09 (1.55) | 3.91 (2.76) |
| Ethnicity, N (%)* | ||
| White; nonwhite | 23 (92%); 2 (8%) | 15 (68%); 7 (32%) |
| Medication status, N (%) | ||
| Psychiatric medications (yes/missing) | 11 (50%); 1 (4%) | 5 (23%); 0 (0%) |
| Acetylcholinesterase inhibitors (yes/missing) | 17 (77%); 1 (4%) | 11 (50%); 0 (0%) |
| Other medications (yes/missing) | 14 (64%); 1 (4%) | 20 (91%); 0 (0%) |
| Caregiver Information | ||
| Caregiver gender: men; women, N (%) | 8 (32%); 16 (64%); missing: 1 (4%) | 11 (50%); 11 (50%) |
| Relationship to patient, N (%)* | ||
| Spouse | 21 (91%) | 12 (55%) |
| Child | 0 (0%) | 6 (27%) |
| Friend | 2 (9%) | 2 (9%) |
| Living with caregiver, N (%) | ||
| Yes; no | 21 (91%); 2 (9%) | 17 (77%); 5 (23%) |
With regard to caregivers, gender is shown in Table 1. There was not a significant association of diagnosis group and whether or not a caregiver lived with the patient: χ2=6.85; NS. All caregivers had contact a minimum of 2–3 times per week with the patient. The majority of caregivers were spouses of the patient, but there was a significant association of caregiver relationship with patient and diagnosis group: χ2=10.44; p=0.015; wherein more LOAD patients also had children, friends, or siblings as caregivers.
Presence of Anxiety Between Diagnosis Groups
Seventy percent of EOAD caregivers, versus 27% of LOAD caregivers, reported the presence of anxiety symptoms in patients, and the group difference was significant (OR: 6.10; p=0.006; Figure 1).
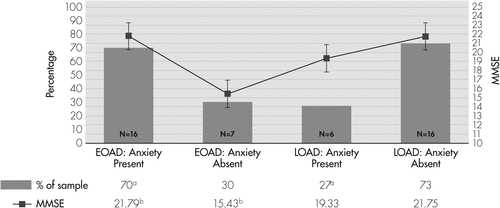
Percentage of individuals with (dark gray bar) and without (light gray bar) anxiety.
aIndicates significant between-group differences with respect to presence/absence of anxiety.
bIndicates significant between-group differences with respect to MMSE score. Error bars represent the standard error. Missing data for Neuropsychiatric Inventory Anxiety: N=2 (8%) for early-onset cohort and N=2 for late-onset cohort (9.1%).
Demographic Associations With Anxiety Within and Between Diagnosis Groups
In the stratified analyses, the male patients in the EOAD group were more likely to experience anxiety (85.7% versus 44.4% for male versus female, respectively; OR: 7.5; 95% CI: 1.02–55.0; p=0.036); the male patients in the LOAD group were also more likely to experience anxiety, but not significantly so (40% versus 0%; OR: 10.3; 95% CI: 0.41–259.3; NS). For the EOAD group, the odds of having anxiety were borderline-associated with a higher MMSE score (OR: 1.18; 95% CI: 1.00–1.39; p=0.05). However, MMSE score was not significantly associated with anxiety outcome for the LOAD group. There were no significant associations between anxiety and age, education, functional status, time since diagnosis, or ethnicity.
We further examined whether the above associations differed by diagnosis group by using a single-model approach. The interaction term (group × gender) was not significant, indicating no evidence that the relationship between anxiety and gender differed by diagnosis group. Thus, we re-fit the model without the interaction term, and found that 1) male gender was associated with a significantly higher odds of having anxiety (OR: 8.50; 95% CI: 1.58–45.8; p=0.013); and 2) the EOAD participants were much more likely to have documented anxiety than the LOAD participants (OR: 9.68; 95% CI: 1.98–47.4; p=0.005). However, we observed a significant interaction of MMSE score and anxiety across levels of diagnosis group (p=0.041), indicating that the relationships between MMSE score and anxiety were different between the groups. As MMSE score increased, the probability of the early-onset group’s having anxiety increased (OR: 1.18; p=0.050), whereas that for the late-onset group (LOAD) decreased (OR=0.84; NS). When the MMSE score reached 24, the suggested cutoff for dementia, the difference in odds of having anxiety was larger (OR: 23.10; p=0.041).
Types of Anxiety Symptoms Endorsed
Table 2 depicts the specific questions asked of caregivers with regard to the types of anxiety symptoms they endorsed in the patient. Results indicated that EOAD patients experienced significantly more anxiety when they were separated from their caregivers than did LOAD patients (34.8% versus 9.5%, respectively; p=0.046). Also, a significant association between sex of the patient and endorsement of separation anxiety was observed (32% versus 6% for male versus female patients, respectively; p=0.049).
| Neuropsychiatric Inventory Anxiety Item | χ2 [df] | % Positive: EOAD | % Positive: LOAD |
|---|---|---|---|
| Does the patient say that he/she is worried about planned events? | 0.577 [1] | 17.3% | 9.5% |
| Does the patient avoid certain places or situations that make him/her more nervous, such as riding in the car, meeting with friends, or being in crowds? | 0.911 [1] | 13% | 4.8% |
| Does the patient become more nervous and upset when separated from you or caregiver? | 3.988 [1]* | 34.8% | 9.5% |
| Does the patient have periods of feeling shaky, unable to relax, or feeling excessively tense? | 1.738 [1] | 17.3% | 4.8% |
| Does the patient have periods of shortness of breath, gasping, or sighing for no apparent reason other than nervousness? | 0.004 [1] | 4.5% | 5% |
| Does the patient complain of “butterflies in his/her stomach” or racing or pounding of the heart in association with nervousness? | 2.295 [1] | 0% | 9.5% |
Psychiatric Symptom Comorbidity: Correlation
When the EOAD and LOAD groups were analyzed collectively, anxiety (as measured by the frequency × severity index) was significantly associated with the other NPI scales, including delusions, irritability, and aberrant motor behavior. Stratified analyses indicated that, within EOAD, anxiety was not significantly correlated with any of the other NPI scales. For LOAD, however, anxiety was significantly associated with delusions, irritability, aberrant motor activity, and agitation. Anxiety was not significantly associated with depression. Table 3 displays the correlational analyses.
| EOAD | LOAD | Combined Groups | |
|---|---|---|---|
| Agitation | 0.09 | 0.44* | 0.23 |
| Apathy | 0.23 | 0.13 | 0.19 |
| Delusions | 0.37 | 0.45* | 0.33* |
| Depression | 0.14 | 0.20 | 0.23 |
| Disinhibition | –0.16 | 0.31 | 0.07 |
| Euphoria | –0.25 | 0.000 | –0.13 |
| Hallucinations | 0.36 | 0.35 | 0.19 |
| Irritability | 0.22 | 0.63** | 0.54** |
| Aberrant Motor Behavior | 0.09 | 0.45* | 0.46* |
Discussion
This study revealed differences in anxiety between the early- and late-onset forms of AD; early-onset patients demonstrated higher levels of anxiety than their late-onset counterparts. The correlates of anxiety were quite different between patients with EOAD and LOAD. The anxiety in early-onset patients was associated with being separated from their caregivers, and it corresponded to higher MMSE scores. In contrast, the anxiety in late-onset patients was associated with comorbid psychiatric and behavioral symptoms as the dementia progressed.
When further comparing EOAD and LOAD groups, there were some notable differences in relationships between demographic variables and anxiety. First, more men were anxious than were women (especially within the early-onset cohort), which is the opposite pattern from that observed in the general population. This finding that men are more anxious was also documented in previous research, but not within an EOAD and LOAD comparison,45 and may be explained among the early-onset cohort by the fact that these men are in midlife, and, according to traditional gender roles, are at the height of their breadwinning career and family responsibilities. Therefore, onset of a neurodegenerative disease at a younger age presents a higher level of challenges and functional impairments than onset at a later age, often during the retirement years. Although these reports of anxiety were from the caregiver, the majority of whom were female spouses, there was no significant association of sex of caregiver with presence/absence of anxiety. Moreover, although there was a significant association of ethnicity with diagnostic group, ethnicity was not significantly associated with presence or absence of anxiety. Finally, it is possible that, given the age difference in the two groups, that anxiety may have been simply related to age. This cannot be entirely excluded; however, previous literature has been mixed with regard to the relationship of age and anxiety in AD.35,36,43,46
Among EOAD patients, those who had endorsed anxiety had higher MMSE scores than those without anxiety. Although we did not specifically measure insight in the current study, other investigations have noted an association of patients’ better global cognitive functioning with greater insight into their situation and disease, which, in turn, is related to greater levels of anxiety.25,26,28 Similarly, EOAD has been termed as a distinct disease process, with different genetic contributors, disease course, neuroimaging findings, neuropathology, and clinical manifestations,47 and patients with EOAD tend to have greater awareness of their disorder and functional burden.47
Because anxiety has been so difficult to define and separate from other common comorbid psychiatric symptomatology,4,7 this study examined the overlap of anxiety with other psychiatric symptoms as measured by the UCLA NPI. Anxiety was associated with other activating and behavioral psychiatric symptoms (e.g., delusions, irritability, aberrant motor behavior, agitation), but only in the LOAD group. Thus, although anxiety may be tied with other activating psychiatric symptoms in older AD patients, it seems to be separate from these symptoms in younger AD patients. Despite the well-established overlap of anxiety and depression,4,7 depression was not correlated in anxiety either within or across groups. This finding has implications for how anxiety might be alleviated in the late-onset group with treatment of some of these positive psychiatric symptoms through the use of behavioral interventions and psychotropic medications.
This study has potential limitations. First, there were no self-report measures of anxiety; rather, the presence or absence of anxiety relied on caregiver report. Cognitive deficits in dementia, however, compromise the value of self-reports. Second, there was no measure of caregiver distress. Although caregiver anxiety may have influenced their reports of patient anxiety, it would not account for some of the differences found in this study, such as worse anxiety with better MMSE scores in the EOAD patients. Finally, this investigation had a moderate sample size. It was intended to characterize anxiety, rather than quantify it, per se, and it can serve as pilot data, upon which future studies, with larger sample sizes, can expand. Such findings, however, have implications for diagnosis, management, and treatment for AD patients suffering from psychiatric comorbidities.
The findings of this study have implication for several practical interventions. For example, when anxiety is present in patients with AD of early onset, clinicians can focus on strategies that alleviate the fear of caregiver separation. In contrast, when anxiety is present in patients with AD of late onset, clinicians may consider a thorough evaluation for other neuropsychiatric symptoms. This study also suggests that men may be at greater risk for psychiatric symptoms in AD than women. Further work is needed in order to characterize the different reasons for anxiety in EOAD and LOAD and the mechanisms for alleviating it.
1 Seignourel PJ, Kunik ME, Snow L, et al: Anxiety in dementia: a critical review. Clin Psychol Rev 2008; 28:1071–1082; E-pub: 2008/06/17; doi: S0272-7358(08)00064-0 [pii] 10.1016/j.cpr.2008.02.008. PubMed PMID: 18555569; PubMed Central PMCID: PMC2575801Google Scholar
2 : Detecting and managing depression and anxiety in people with demenia. Curr Opin Psychiatry 2000; 13:55–59Crossref, Google Scholar
3 Chemerinski E, Petracca G, Manes F, et al: Prevalence and correlates of anxiety in Alzheimer's disease. Depress Anxiety 1998; 7:166–170; E-pub: 1998/08/26. doi: 10.1002/(SICI)1520-6394(1998)7:4<166::AID-DA4>3.0.CO;2-8 [pii]. PubMed PMID: 9706453Google Scholar
4 Ferretti L, McCurry SM, Logsdon R, et al: Anxiety and Alzheimer's disease. J Geriatr Psychiatry Neurol 2001; 14:52–58; E-pub: 2001/04/03; PubMed PMID: 11281317Google Scholar
5 Forsell Y, Winblad B: Anxiety disorders in non-demented and demented elderly patients: prevalence and correlates. J Neurol Neurosurg Psychiatry 1997; 62:294–295; E-pub: 1997/03/01; PubMed PMID: 9069497; PubMed Central PMCID: PMC1064171Google Scholar
6 : The prevalence of psychotic, depressive, and anxiety syndromes in demented and non-demented 85-year-olds. Int J Geriatr Psychiatry 1993; 15:42–49Google Scholar
7 Starkstein SE, Jorge R, Petracca G, et al: The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry 2007; 15:42–49; E-pub: 2006/12/30. doi: 15/1/42 [pii] 10.1097/01.JGP.0000229664.11306.b9. PubMed PMID: 17194814Google Scholar
8 Ballard C, Neill D, O'Brien J, et al: Anxiety, depression, and psychosis in vascular dementia: prevalence and associations. J Affect Disord 2000; 59:97–106; E-pub: 2000/06/06. doi: S0165-0327(99)00057-9 [pii]. PubMed PMID: 10837878Google Scholar
9 : Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: The Cache County Study. Int J Geriatr Psychiatry 2001; 16:1043–1053Crossref, Medline, Google Scholar
10 Wands K, Merskey H, Hachinski VC, et al: A questionnaire investigation of anxiety and depression in early dementia. J Am Geriatr Soc 1990; 38:535–538; E-pub: 1990/05/01; PubMed PMID: 2332576Google Scholar
11 Aarsland D, Cummings JL, Larsen JP: Neuropsychiatric differences between Parkinson's disease with dementia and Alzheimer's disease. Int J Geriatr Psychiatry 2001; 16:184–191; E-pub: 2001/03/10; doi: 10.1002/1099-1166(200102)16:2<184::AID-GPS304>3.0.CO;2-K [pii]. PubMed PMID: 11241724Google Scholar
12 Sultzer DL, Levin HS, Mahler ME, et al: A comparison of psychiatric symptoms in vascular dementia and Alzheimer's disease. Am J Psychiatry 1993; 150:1806–1812; E-pub: 1993/12/01; PubMed PMID: 8238634Google Scholar
13 Lyketsos CG, Steinberg M, Tschanz JT, et al: Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157:708–714; E-pub: 2000/04/28; PubMed PMID: 10784462Google Scholar
14 Porter VR, Buxton WG, Fairbanks LA, et al: Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. J Neuropsychiatry Clin Neurosci 2003; 15:180–186; E-pub: 2003/05/02; PubMed PMID: 12724459Google Scholar
15 Harwood DG, Ownby RL, Barker WW, et al: The Behavioral Pathology in Alzheimer's Disease Scale (BEHAVE-AD): factor structure among community-dwelling Alzheimer's disease patients. Int J Geriatr Psychiatry 1998; 13:793–800; E-pub: 1998/12/16; doi: 10.1002/(SICI)1099-1166(1998110)13:11<793; AID-GPS875>3.0.CO;2-Q [pii]; PubMed PMID: 9850876Google Scholar
16 Mendez MF, Martin RJ, Smyth KA, et al: Psychiatric symptoms associated with Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1990; 2:28–33; E-pub: 1990/01/01; PubMed PMID: 2136060Google Scholar
17 Sultzer DL, Levin HS, Mahler ME, et al: Assessment of cognitive, psychiatric, and behavioral disturbances in patients with dementia: the Neurobehavioral Rating Scale. J Am Geriatr Soc 1992; 40:549–455; E-pub: 1992/06/01; PubMed PMID: 1587970Google Scholar
18 Tariot PN, Mack JL, Patterson MB, et al: The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer's Disease: The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer's Disease. Am J Psychiatry 1995; 152:1349–1357; E-pub: 1995/09/01; PubMed PMID: 7653692Google Scholar
19 Teri L, Truax P, Logsdon R, et al: Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist. Psychol Aging 1992; 7:622–631; E-pub: 1992/12/01; PubMed PMID: 1466831Google Scholar
20 Tractenberg RE, Patterson M, Weiner MF, et al: Prevalence of symptoms on the CERAD behavior rating scale for dementia in normal elderly subjects and Alzheimer's disease patients. J Neuropsychiatry Clin Neurosci 2000; 12:472–479; E-pub: 2000/11/18; PubMed PMID: 11083164.Google Scholar
21 Jost BC, Grossberg GT: The evolution of psychiatric symptoms in Alzheimer's disease: a natural history study. J Am Geriatr Soc 1996; 44:1078–1081; E-pub: 1996/09/01; PubMed PMID: 8790235Google Scholar
22 Ownby RL, Harwood DG, Barker WW, et al: Predictors of anxiety in patients with Alzheimer's disease. Depress Anx 2000; 11:38–42; E-pub: 2000/03/21. doi: 10.1002/(SICI)1520-6394(2000)11:1<38::AID-DA6>3.0.CO;2-E [pii]; PubMed PMID: 10723634Google Scholar
23 Teri L, Ferretti LE, Gibbons LE, et al: Anxiety of Alzheimer's disease: prevalence and comorbidity. J Gerontol A Biol Sci Med Sci 1999; 54:M348–M352; E-pub: 1999/08/26; PubMed PMID: 10462166Google Scholar
24 Tsang SW, Lai MK, Francis PT, et al: Serotonin transporters are preserved in the neocortex of anxious Alzheimer's disease patients. Neuroreport 2003; 14:1297–1300; E-pub: 2003/07/24; doi: 10.1097/01.wnr.0000077546.91466.a8; PubMed PMID: 12876460Google Scholar
25 : Unawareness of memory deficit in Alzheimer's disease: relation to mood and other disease variables. Neuropsychiatry Neuropsychol Behav Neurol 1995; 8:176–181Google Scholar
26 Derouesne C, Thibault S, Lagha-Pierucci S, et al: Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. Int J Geriatr Psychiatry 1999; 14:1019–1030; E-pub: 1999/12/23. doi: 10.1002/(SICI)1099-1166(199912)14:12<1019::AID-GPS61>3.0.CO;2-F [pii]; PubMed PMID: 10607969Google Scholar
27 Loring SH, Mead J, Griscom NT: Dependence of diaphragmatic length on lung volume and thoracoabdominal configuration. J Appl Physiol 1985; 59:1961–1970; E-pub: 1985/12/01; PubMed PMID: 4077804Google Scholar
28 Papanicolaou AC, Loring DW, Eisenberg HM: Probe-evoked potential correlates of hemispheric asymmetries during a speech shadowing task. Int J Neurosci 1985; 28(1–2):83–89; E-pub: 1985/10/01; PubMed PMID: 4066194Google Scholar
29 Mayeux R, Stern Y, Spanton S: Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology 1985; 35:453–461; E-pub: 1985/04/01; PubMed PMID: 3982631Google Scholar
30 Seltzer B, Sherwin I: A comparison of clinical features in early- and late-onset primary degenerative dementia: one entity or two? Arch Neurol 1983; 40:143–146; E-pub: 1983/03/01; PubMed PMID: 6830452Google Scholar
31 : Nonamestic presentations of early-onset Alzheimer’s disease. Am J Alz Dis Other Dement 2012; 27:413–420Google Scholar
32 Koedam EL, Lauffer V, van der Vlies AE, et al: Early- versus late-onset Alzheimer's disease: more than age alone. J Alzheimers Dis 2010; 19:1401–1408; E-pub: 2010/01/12; doi: 04U02703PHW3K242 [pii] 10.3233/JAD-2010-1337; PubMed PMID: 20061618Google Scholar
33 Smits LL, Pijnenburg YA, Koedam EL, et al: Early-onset Alzheimer's disease is associated with a distinct neuropsychological profile. J Alzheimers Dis 2012; 30:101–108; E-pub: 2012/03/01; doi: MM150626L2P5G072 [pii] 10.3233/JAD-2012-111934; PubMed PMID: 22366769Google Scholar
34 : The early-onset dementias: a study of clinical characteristics and service use. J Geriatr Psychiatry 1996; 11:863–869Crossref, Google Scholar
35 : Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer's disease. Int J Geriatr Psychiatry 2007; 22:896–901Crossref, Medline, Google Scholar
36 van Vliet D, de Vugt ME, Aalten P, et al: Prevalence of Neuropsychiatric Symptoms in Young-Onset Compared to Late-Onset Alzheimer's Disease, Part 1: findings of the two-year longitudinal NeedYD Study. Dement Geriatr Cogn Disord 2012; 34(5–6):319–327; E-pub: 2012/12/05; doi: 10.1159/000342824 000342824 [pii]; PubMed PMID: 23208452Google Scholar
37 Bungener C, Jouvent R, Derouesne C: Affective disturbances in Alzheimer's disease. J Am Geriatr Soc 1996; 44:1066–1071; E-pub: 1996/09/01; PubMed PMID: 8790232Google Scholar
38 Kaiser NC, Melrose RJ, Liu C, et al: Neuropsychological and neuroimaging markers in early- versus late-onset Alzheimer's disease. Am J Alz Dis Other Demen 2012; 27:520–529; E-pub: 2012/09/20; doi: 10.1177/1533317512459798 1533317512459798 [pii]; PubMed PMID: 22990206Google Scholar
39 McKhann G, Drachman D, Folstein M, et al: Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS-ADRDA Work Group Under the Auspices of the Dept. of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944; E-pub: 1984/07/01; PubMed PMID: 6610841Google Scholar
40 Crum RM, Anthony JC, Bassett SS, et al: Population-based norms for the Mini-Mental State Exam by age and educational level. JAMA 1993; 269:2386–2391; E-pub: 1993/05/12; PubMed PMID: 8479064Google Scholar
41 Galasko D, Bennett D, Sano M, et al: An inventory to assess activities of daily living for clinical trials in Alzheimer's disease: The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997; 11(Suppl 2):S33–S39; E-pub: 1997/01/01; PubMed PMID: 9236950Google Scholar
42 Cummings JL, Mega M, Gray K, et al: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314; E-pub: 1994/12/01; PubMed PMID: 7991117Google Scholar
43 Cummings JL: The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997; 48:(Suppl 6):S10–S16; E-pub: 1997/05/01; PubMed PMID: 9153155Google Scholar
44 Fuh JL, Liu CK, Mega MS, et al: Behavioral disorders and caregivers' reaction in Taiwanese patients with Alzheimer's disease. Int Psychogeriatr 2001; 13:121–128; E-pub: 2001/05/16; PubMed PMID: 11352329Google Scholar
45 Calleo JS, Kunik ME, Reid D, et al: Characteristics of generalized anxiety disorder in patients with dementia. Am J Alz Dis Other Demen 2011; 26:492–497; E-pub: 2011/11/09; doi: 10.1177/1533317511426867 1533317511426867 [pii]; PubMed PMID: 22062223; PubMed Central PMCID: PMC3252749Google Scholar
46 : Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. J Neuropsychiatry Clin Neurosci 2003; 8:180–186Crossref, Google Scholar
47 Mendez MF: Early-onset Alzheimer's disease: nonamnestic subtypes and Type 2 AD. Arch Med Res 2012; 43:677–685; E-pub: 2012/11/28; doi: 10.1016/j.arcmed.2012.11.009 S0188-4409(12)00337-2 [pii]; PubMed PMID: 23178565; PubMed Central PMCID: PMC3532551Google Scholar



