Conventional SPECT Versus 3D Thresholded SPECT Imaging in the Diagnosis of ADHD: A Retrospective Study
Abstract
Brain single photon emission CT (SPECT) scans indirectly show functional activity via measurement of regional cerebral blood flow. In conventional SPECT scans, the typical tomographic slices are produced. In three-dimensional thresholded SPECT scans, pixels representing activity below a certain threshold are discarded. A retrospective analysis of 427 patients shows that three-dimensional thresholded SPECT scans yield a sensitivity for predicting clinical attention deficit hyperactivity disorder of 54% [95% confidence interval (CI), 46%−61%; specificity, 76%; 95% CI, 71%−81%] compared with 4% sensitivity [95% CI, 2%−8%; specificity, 97%; 95% CI, 94%−98%] for conventional SPECT scans. For 170 of the patients originating from a general psychiatry practice, conventional SPECT showed 10% sensitivity (95% CI, 4%−23%) and 98% specificity (95% CI, 93%−99%), whereas three-dimensional thresholded SPECT showed 83% sensitivity (95% CI, 68%−91%) and 77% specificity (95% CI, 69%−83%). These findings indicate that a much stronger signal is obtained when the three-dimensional thresholded SPECT scan is performed rather than the conventional SPECT scan in detecting attention deficit hyperactivity disorder and suggest similar results may be obtained for other psychiatric disorders.
There is controversy whether neuroimaging is clinically useful in the diagnosis and management of attention deficit hyperactivity disorder (ADHD) and other psychiatric disorders.1–3 Single photon emission CT (SPECT) is a well-known method of neuroimaging thought to show functional activity indirectly via measurement of relative perfusion.4–8 Visualizing cerebral functional activity may allow better diagnosis and more targeted effective treatment of various psychiatric disorders.9–16
Lee and colleagues reported that children with clinical ADHD show reduced orbitofrontal regional cerebral blood flow on SPECT scan. Treatment with methylphenidate provides improvement of clinical symptoms, as well as normalizing the reduced orbitofrontal activity.17 Functional MRI (fMRI) also shows dysfunction in the prefrontal cortex in children with ADHD, which again shows a trend toward normalization with methylphenidate treatment.18,19 Other work shows hypoperfusion in the orbitofrontal regions by SPECT scan of both children and adults with clinical ADHD.9,11,20
Here we present a naturalistic retrospective study where we look for the presence of orbitofrontal hypoperfusion on SPECT scans and consider the sensitivity and the specificity of such findings in predicting which patients have a clinical DSM-IV diagnosis of ADHD.
Methods
Brain SPECT scans were offered to patients of community-based psychiatric clinics and offices presenting with a full spectrum of psychiatric and neuropsychiatric disorders and ranging in age from teenage to geriatric. Scans were not offered for straightforward cases, but only where cases were partially or fully refractory in response to treatment or where diagnoses were complex. Otherwise there was no selection of patients. Scans were performed at a Toronto, Canada, tertiary care hospital and followed the guidelines established by the Society of Nuclear Medicine.21
The patient received 10–20 millicuries of Tc99m-hexamethylpropylene-amine oxime or in some cases Tc99m-ethyl cysteinate dimer.21 There was a radiation exposure of 3–6 mSv to the patient, which is comparable to a year or so of background radiation, which has not been shown to cause harm to humans,22 although the debate with regard to safety continues because significantly higher radiation exposures are known to cause harm.23
In what is termed here a “baseline” scan, the patient rested quietly in a semidarkened room prior to and for at least 2 minutes after the injection of the radiotracer. In a “concentration” scan, the patient was asked questions that stimulated prefrontal cortical activity prior to and for at least 2 minutes after the injection of the radiotracer. There is evidence that such stimulation in a patient with ADHD may decrease perfusion in the orbitofrontal cortex,24–26 although other evidence shows that there may be increased activity in the right dorsolateral prefrontal cortex, particularly with response inhibition stimulation.27 Within 90 seconds after administration of the radiotracer, there is uptake and retention by different regions of the brain, in approximate proportion to the blood flow to the regions. As the technetium-99m decays, these different regions of the brain will thus then emit different rates of gamma decay photons, in proportion to the regional cerebral blood flow (rCBF) at the time of injection. The relationship between rCBF and regional cerebral activity is the basis for perfusion SPECT.4–7
After injection of the radiotracer, there is a waiting period of 30–60 minutes. The patient’s head is then imaged by gamma photon cameras, and a three-dimensional (3D) cloud of pixels representing the gamma radiation from the brain is registered. This cloud of pixels is corrected for attenuation and filtered. It is sliced into orthogonal planes to produce the tomograms of what is termed here a “conventional SPECT” scan. An example of conventional SPECT images (using Tc99m-hexamethylpropylene-amine oxime) of a patient with clinical ADHD is shown in Figure 1. The pixel cloud is then thresholded at a predetermined level (in this study only areas of activity equal to or exceeding 55% of the most active area are kept) and rendered into a solid 3D form of what is termed here a “3D thresholded SPECT” scan. An example of a 3D thresholded SPECT image of the same patient with ADHD corresponding to the conventional tomographic slices of Figure 1 is shown in Figure 2. Note that the orbitofrontal perfusion defects (i.e., hypoperfusion) are readily seen in the 3D thresholded image. This is just one view of the 3D image, which can be more fully seen at all angles on a computer workstation.
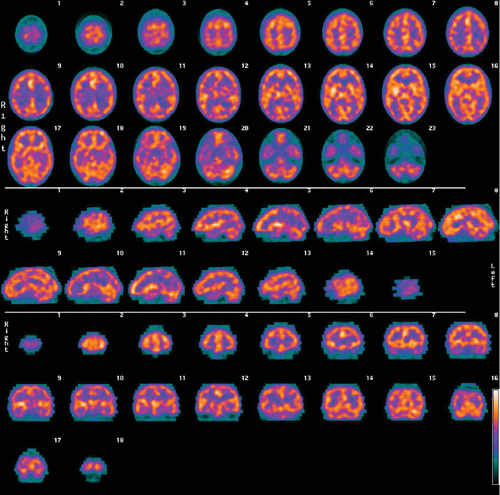
FIGURE 1. Conventional SPECT Brain Scan Images of a Patient With Clinical ADHDa
a ADHD: attention deficit hyperactivity disorder; SPECT: single photon emission CT.
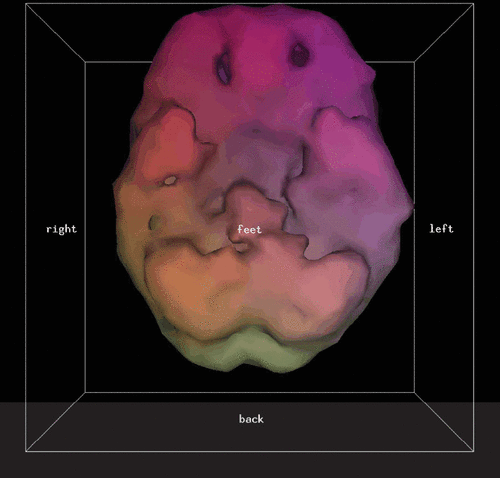
FIGURE 2. Underside View of Brain of Three-Dimensional Thresholded SPECT Scan Image of the Same Patient Shown in Figure 1a
a Defects (hypoperfusion) in the orbitofrontal regions of the prefrontal cortex are readily observable. SPECT: single photon emission CT.
Scanning Procedures
Most of the scans involved using Tc99m-hexamethylpropylene-amine oxime radiotracer, but due to supply issues, Tc99m-ethyl cysteinate dimer radiotracer was used in 2.6% of patients. There was no relationship to clinical diagnosis or other patient factors with regard to who received which radiotracer. Both radiotracers are approximately equally sensitive for showing prefrontal cortical defects, although the Tc99m-ethyl cysteinate dimer radiotracer tends to be more sensitive for showing temporal lobe defects.28,29
Sixteen percent of patients received concentration scans, i.e., prior to and after injection of the radiotracer, the patient performs mental activities intended to fatigue directed attention capacity. In a concentration scan, the patient was asked to perform progressively harder serial subtractions, as well as spelling words backward and repeating numbers backward until mistakes were being made, and to continue at this level while being interrupted with new subtractions, numbers, and words for 60 seconds prior to the injection of the radiotracer and for at least 2 minutes after injection. Because concentration scans are thought to bring out ADHD features on SPECT scans,18,24–26 when ADHD or the prefrontal cortical system involvement was suspected in a patient, a concentration scan was often ordered, although this was not done consistently. This variable is handled in the Results section below.
A small intravenous saline line was started prior to administration of the radiotracer, so that the injection of the radiotracer would not be associated with the pain of intravenous insertion. The protocol of either a baseline scan or a concentration scan, as described above, was then followed with regard to injection of the radiotracer. Then the patient waited for an additional 30–60 minutes in the waiting room to allow back diffusion from nonneural structures. The patient’s head was then scanned with a Picker Prism 3000 three-headed camera. The three heads underwent 120 steps, 3° per step, 128 × 128 data matrix, and continuous acquisition. Approximately 22–30 seconds were spent on each step giving a total scan time of approximately 15–20 minutes. A low-energy ultra-high-resolution fan beam collimator was used, and approximately 3–4 million counts in total were obtained. The data then passed through a ramp backprojection filter followed by a 3D Butterworth filter (order, 5.0; cutoff frequency, 0.2–0.3 cycle per pixel based on the mean counts/pixel). Image voxels outside the head/brain region were set to zero by applying a mask to the reconstructed transaxial images. 3D SPECT images were generated via Picker Prism Odyssey Rendering Software.
Image Processing
From the scan data, conventional SPECT images (i.e., the conventional tomographic slices) and 3D thresholded SPECT images (i.e., the thresholded 3D images) were produced for each patient. A 55% setting was used for the threshold; pixels representing areas of activity less than 55% of the highest area were discarded in the production of the 3D image. This threshold reflects the early work of Mena and colleagues30–32 and has been used by Amen24 in tens of thousands of SPECT scans.
Statistical parametric mapping is a statistical technique often applied to neuroimaging to statistically show differences in regional cerebral activity.33 We intended to process all images with statistical parametric mapping but made the active decision not to for the following reasons:
| 1. | although existing statistical parametric mapping software technically worked with our conventional SPECT image data, it was not technically compatible with the 3D thresholded SPECT image data; | ||||
| 2. | the manual time involved and consequent expense of skilled human labor in applying the statistical parametric mapping software to the image data would not allow the SPECT scans to be justified as a clinical procedure, certainly not a community clinical procedure, in the environment of the local current health care system; and | ||||
| 3. | statistical parametric mapping is theoretically able to delineate areas of decreased perfusion on conventional SPECT scans but proved problematic with our data due to a lack of an adequate normative database matched for age, radiotracer and scanner. | ||||
The conventional SPECT scans and the 3D thresholded SPECT scans were then read by the same nuclear medicine physician. Although this physician was not formally blinded to the clinical information, an effort was made to read the images solely on what the scans showed, without influence of the clinical descriptions, and a neuroanatomical rather than a neuropsychiatric report was produced. For every patient, the conventional images were read first, and then the 3D thresholded images were read; in this way, the reading of the 3D thresholded SPECT scan did not influence the reading of the conventional SPECT scan.
Results
Study Patient Population
Where scans were offered, virtually all patients (approximately 95%) accepted the scans. Risks (radiation exposure, limited literature on use of results in psychiatry) were carefully explained to all patients, and informed consent was obtained. For patients younger than 18 years of age and for patients not able to make a fully informed decision, additional informed consent from a caregiver was obtained. A total of 427 patients received brain SPECT scans. The average age of the patients was 40.9 years (SD=15.7), and 51.1% of the patients were women; 6.1% of the patients were younger than 18 years of age (average age, 14.1 years old; SD=2.7); and 39.8% of patients had a clinical DSM-IV diagnosis of ADHD. Four physicians working at different community-based offices and clinics ordered the brain SPECT scans for these 427 patients. A description of each physician practice is given in Table 1.
| Type of Practice | SPECT Scans–Number of Patients | Mean Age and Standard Deviation | Percent Female (%) |
|---|---|---|---|
| General adult psychiatric practice, all patients referred from other physicians “general psychiatry practice” | 170 | 40.2 years, SD=15.0 years | 43 |
| Adult psychiatric practice with emphasis on fibromyalgia, chronic fatigue “psychiatry including fibromyalgia practice” | 139 | 46.8 years, SD=13.9 years | 63 |
| Child and adult psychiatric practice, with specialization in ADHD and bipolar II disorder “child and adult psychiatry practice” | 105 | 33.6 years, SD=16.5 years | 43 |
| Adult medical psychotherapy practice “psychotherapy practice” | 13 | 45.1 years, SD=10.4 years | 92 |
| Total | 427 | 40.9 years, SD=15.7 years | 51 |
TABLE 1. Description of Psychiatric Practices From Which Patients Originatea
Sensitivities and Specifities
The literature reports that defects (reduced rCBF) in the orbitofrontal regions are associated with ADHD.9,11,17–20,26,27,34,35 Thus, in this retrospective study, we decided to compare SPECT findings of defects in the orbitofrontal regions with clinical DSM-IV diagnoses of ADHD.36 As noted, both conventional SPECT images and 3D thresholded SPECT images were created from the scan data for every patient.
If the assumption is made that the DSM-IV diagnosis is the valid variable, then it is possible to calculate sensitivities and specificities of the SPECT scans for predicting a DSM-IV diagnosis of ADHD, as shown in Table 2. For all 427 patients, the 3D thresholded SPECT scan gave a sensitivity of 54% [95% confidence interval (CI) of 46%−61%] and a specificity of 76% (95% CI of 71%−81%) for the DSM-IV diagnosis of ADHD. In comparison, the conventional SPECT scan gave a sensitivity of 4% (95% CI of 2%−8%) and a specificity of 97% (95% CI of 94%−98%) for the DSM-IV diagnosis of ADHD.
| SPECT scan is considered positive if defects (hypoperfusion) in the orbitofrontal cortex are seen | Sensitivity (95% Confidence Interval) | Specificity (95% Confidence Interval) | Positive Predictive Value (95% Confidence Interval) |
|---|---|---|---|
| Conventional SPECT versus clinical ADHD (all physicians’ patients) | 4.1% (2%–8%) | 96.5% (94%–98%) | 43.8% (23%–67%) |
| General psychiatry practice patients | 10.0% (4%–23%) | 97.7% (93%–99%) | 57.1% (25%–84%) |
| Psychiatry including fibromyalgia practice patients | 2.0% (0.3%–10%) | 93.2% (86%–97%) | 14.3% (3%–51%) |
| Child and adult Psychiatry practice patients (with ADHD and bipolar II specialization) | 2.5% (1%–9%) | 100% (87%–100%) | 100% (34%–100%) |
| 3D thresholded SPECT versus clinical ADHD (all physicians’ patients) | 53.5% (46%–61%) | 76.3% (71%–81%) | 59.9% (52%–67%) |
| General psychiatry practice patients | 82.5% (68%–91%) | 76.9% (69%–83%) | 52.4% (40%–64%) |
| Psychiatry including fibromyalgia practice patients | 41.2% (29%–55%) | 71.6% (61%–80%) | 45.7% (32%–60%) |
| Child and adult Psychiatry practice patients (with ADHD and bipolar II specialization) | 46.8% (36%–58%) | 96.2% (81%–99%) | 97.4% (86%–100%) |
| 3D thresholded SPECT versus clinical ADHD (all physicians’ patients but only where concentration scan done) (16.1% of total patients) | 66.7% (52%–79%) | 79.2% (60%–91%) | 85.7% (71%–93%) |
| General psychiatry practice patients | 70.8% (51%–85%) | 88.2% (66%–97%) | 89.5% (69%–97%) |
| Psychiatry including fibromyalgia practice patients | 100% (51%–100%) | 0.0% (0%–79%) | 80.0% (38%–96%) |
| Child and adult Psychiatry practice patients (with ADHD and bipolar II specialization) | 52.9% (31%–74%) | 100% (51%–100%) | 100% (70%–100%) |
| 3D thresholded SPECT versus clinical ADHD (all physicians’ patients but only where scan performed prior to psychotropic use) (33.7% of total patients) | 68.7% (57%–79%) | 84.4% (75%–91%) | 79.3% (67%–88%) |
| General psychiatry practice patients | 86.2% (69%–95%) | 87.3% (77%–93%) | 75.8% (59%–87%) |
| Psychiatry including fibromyalgia practice patients | 33.3% (6%–79%) | 0.0% (0%–79%) | 50.0% (10%–91%) |
| Child and adult Psychiatry practice patients (with ADHD and bipolar II specialization) | 57.1% (41%–72%) | 90.0% (60%–98%) | 95.2% (77%–99%) |
| Conventional SPECT versus clinical ADHD (all physicians’ patients<18 years) (6.1% total patients) | 5.3% (1%–25%) | 100% (65%–100%) | 100% (21%–100%) |
| 3D thresholded SPECT versus clinical ADHD (all physicians’ patients<18 years) (6.1% total patients) | 42.1% (23%–64%) | 85.7% (49%–97%) | 88.9% (57%–98%) |
TABLE 2. Sensitivities, Specificities, and Positive Predictive Values for Findings of Orbitofrontal Cortical Defects on the SPECT Scan and DSM-IV ADHD Diagnosesa
Looking more closely at the sensitivity results, from Table 2, it can be seen that the sensitivities for predicting ADHD from the conventional SPECT scans are inferior in all the different types of psychiatric practices compared with the sensitivities obtained from the 3D thresholded SPECT scans. For the diagnosis of ADHD, in the study presented here, there is found to be a large difference in the sensitivity of 3D thresholded SPECT scans compared with the sensitivity of conventional SPECT scans.
Medications and SPECT Scanning
An issue that must be considered in performing SPECT scans for psychiatric patients is the use of psychotropic medications at the time of the scan, as these medications can influence findings. For example, there is evidence that defects in the orbitofrontal cortex normalize after treatment for ADHD.17,19,24 Data from the 427 patients’ files was analyzed to see which patients were on psychotropic medication (stimulants, antidepressants, antipsychotics, and so on) at the time of the scan. If a patient was not on a psychotropic medication for at least five half-lives before the time of the scan, then the patient was considered to be off psychotropic medication. A total of 144 of the 427 patients were found to be off medication at the time of their SPECT brain scan. As shown in Table 2, considering the 3D thresholded SPECT scans for these 144 patients, there was a 69% sensitivity (95% CI, 57%−79%) and an 84% specificity (95% CI, 75%−91%) in predicting whether a patient would have a clinical diagnosis of ADHD. For the patients off psychotropic medications originating from the general psychiatry practice, the sensitivity was 86% (95% CI, 69%−95%) and the specificity was 87% (95% CI, 77%−93%).
The subset of patients from the psychiatry including fibromyalgia practice who were off medication prior to scan was very small and did not include any patients who were considered true negative, i.e., a 3D SPECT scan that did not have defects in the orbitofrontal cortex plus the patient did not have a clinical diagnosis of ADHD. Because specificity=true negatives/(true negatives+false positives), it is shown as zero in Table 2. A similar calculation arose for this practice regarding the small subset of patients who received concentration scans.
Concentration Versus Baseline Scans
As mentioned above, in a baseline scan, the patient is resting in a quiet room when the radiotracer is injected. In a concentration scan, the patient is being asked questions that stimulate prefrontal cortical activity and attempt to fatigue directed attention capacity prior to and after injection of the radiotracer. Whether a baseline or a concentration scan is performed, both conventional SPECT images and 3D thresholded SPECT images are then produced from the scan data.
Sixty-nine patients, 16% of the total, received a concentration scan. In this group for the 3D thresholded images, the sensitivity increased to 67%, and the specificity increased to 79%, compared with a 54% sensitivity and a 76% specificity for all 427 patients scanned. Looking closer at the figures in Table 2 for the concentration scans, the values for the patients originating from psychiatry including fibromyalgia and child and adult psychiatry practices show very wide 95% CIs. In the psychotherapy practice, there were no patients clinically diagnosed with ADHD. Looking at the remaining patients originating from the general psychiatry practice, the sensitivity of the concentration scan is 71% (95% CI, 51%−85%) and the specificity is 88% (95% CI, 66%−97%) compared with an overall 83% sensitivity and 77% specificity for this practice. As discussed earlier in the Methods section, the ordering of the concentration scans by clinicians in this study was not uniform. As well, the attempt to fatigue directed attention capacity was only for 60 seconds prior to injection of the radiotracer. Under these conditions in this study, the concentration scan did not appear to increase the sensitivity for detecting clinical ADHD, although it may have improved specificity.
Subpopulation of Patients With a Clinical Diagnosis of ADHD
If we consider only the 39.8% of patients with a clinical DSM-IV diagnosis of ADHD and disregard the other patients, then what would the sensitivities and the specificities be for these ADHD patients? Because every one of these patients has a diagnosis of ADHD, there will be no true negatives or false positives. Because sensitivity=true positives/(true positives+false negatives), the sensitivities will remain unchanged and will be the same as shown in Table 2. However, specificities cannot be calculated now because specificity=true negatives/(true negatives+false positives) and thus becomes an undefined quantity.
Discussion
Within the psychiatric community, there are enthusiastic proponents to clinically use SPECT scanning for psychiatric patients, and there are equally determined opponents.2,3 In the April-June 2012 issue of the Journal of Psychoactive Drugs,37 Amen and colleagues write: “SPECT has the potential to add clinically meaningful information to enhance patient care beyond current assessment tools…” However, in the July 2012 Consensus Report of the APA Work Group on Neuroimaging Markers of Psychiatric Disorders,38 the first paragraph of the report states: “…there are currently no brain imaging biomarkers that are currently clinically useful for any diagnostic category in psychiatry.” From the work of the present study, an important reason for the discrepancy between these two viewpoints is the failure to distinguish 3D thresholded SPECT scans from conventional SPECT scans. The bulk of the literature on SPECT imaging, whether the images are read with or without the aid of statistical parametric mapping, does not use thresholding of the image data. The 3D thresholded SPECT scan images are mathematically different than the conventional SPECT images: information has been irreversibly thresholded from the images. The thresholded images actually contain less data than the conventional images, but the experimental results in the present study show that this data transformation allows for a better sensitivity in the diagnosis of ADHD. In the present study in the general psychiatry practice, 3D thresholded SPECT scans gave a sensitivity of 83% in predicting which patients would have a clinical diagnosis of ADHD compared with a sensitivity of 10% for the conventional SPECT scans. A 10% sensitivity hardly makes SPECT scans useful for clinical psychiatric applications, in keeping with the viewpoint of the opponents of this technology, whereas the 83% sensitivity for the 3D thresholded SPECT scans gives a diametrically opposite viewpoint, that with 3D thresholding, SPECT is indeed a useful procedure for certain psychiatric patients.
Although the conventional SPECT scans yielded low sensitivities, they produced high specificities for predicting whether a patient would have a clinical diagnosis of ADHD. As can be seen from Table 2, for all patients scanned, the conventional SPECT scan gave a specificity of 97%. The conventional SPECT scans were usually read negative for orbitofrontal cortical hypoperfusion. When a positive finding was occasionally found with conventional SPECT, this most likely corresponded to significant clinical prefrontal cortical dysfunction, in which case a clinical diagnosis of ADHD was often present.
From Bayesian probability theory,39 it is known that medical biomarker tests do not give absolute results, but rather, a test’s performance depends on the prevalence of the value being measured in the population being tested. Looking at Table 2, significant differences in the positive predictive value of the SPECT scans in predicting which patients will have a DSM-IV diagnosis of ADHD are found among the different psychiatric practices from which patients in this study originate. (No patients originating from the psychotherapy practice had a DSM-IV diagnosis of ADHD, and therefore no separate analysis was made for this practice. However, the true negative and false positive results from this practice do affect overall specificity.) Sensitivities and specificities were also calculated for the 26 patients (6.1% of total patients) who were younger than 18 years old. The 3D thresholded SPECT scan gave a sensitivity of only 42% (95% CI of 23%−64%) for this group. Although many of the patients in this group (as well as other patients in this study) had a clinical diagnosis of ADHD, many had been refractory to treatment and their symptoms such as impulsivity and poor attention may have been due to other comorbid or predominant psychopathologies. An increase in what are scored as false negatives will decrease sensitivity because sensitivity=true positives/(true positives+false negatives).
A major limitation of this study is that the clinical DSM-IV diagnosis was accepted as the reference to which to measure the SPECT scans’ predictions to. Unlike in other areas of medicine where more reliable standards exist (e.g., histopathology can be used as a more reliable reference in a study of the usefulness of SPECT scan predictions with regard to the diagnosis of Alzheimer’s disease14,40,41), in psychiatry there are few other biomarkers to use. One way to overcome this limitation in future studies is to consider the SPECT scans’ predictions with respect to response to medication and to outcome, in addition to clinical diagnosis.
Another major limitation of this study was the lack of automated interpretation of the SPECT scans. Although cost-effective efficient reading of the 3D thresholded SPECT scans by a human physician bodes well for implementing this technology in the community, from a scientific point of view, it would have been advantageous to have the images scored by machine, for example, statistical parametric mapping software. There is a need to create an adequate normative database matched for age and radiotracer and that can be used with the 3D thresholded-generated data.
An area that requires additional investigation is the protocol used prior to and immediately following injection of the radiotracer. In this study either the patient was told to simply rest in a semidarkened room prior to and immediately after injection of the radiotracer or the patient underwent the concentration scan protocol described above. There is the need to not just replace the concentration scan protocol with a more standardized one for ease of replication but to investigate the effect of a variety of protocols on the sensitivity and specificity of predicting clinical ADHD based on the SPECT scan results. As well, there is the need to evaluate the utility of two different scans a day apart. If the concentration scan is effective in producing changes in the ADHD patient’s prefrontal cortical functioning, then comparing it to the baseline scan may be useful.9
In the present study, there was only a simplistic consideration of orbitofrontal hypoperfusion on the SPECT scan. This study did not take into account other findings on the SPECT scan that could be used to better correlate with particular diagnoses.34,35 It is known that functional abnormalities occur in ADHD patients in regions away from the prefrontal cortex.26,35 For example, patients with ADHD often show cerebellar hypoperfusion on SPECT scan.24,35 Other diagnostic etiologies, e.g., head trauma, schizophrenia, and fibromyalgia, for example, are also associated with prefrontal cortical defects. However, these disorders have patterns on SPECT scan different than what is seen for ADHD.13,16,42,43 A patient with ADHD and a patient with fibromyalgia may both have orbitofrontal cortical defects, but the fibromyalgia patient will typically have other significant findings.16,42 Defects (i.e., hypoperfusion) in the orbitofrontal cortex in patients with fibromyalgia (or other conditions) will cause an increase in the scoring of patients as false positives, which, limiting the examination of the SPECT scan to orbitofrontal cortical perfusion as in the present study, will decrease the specificity of the 3D SPECT scan in predicting clinical ADHD.
Conclusions
Our findings suggest that, for ADHD, and by inference for other psychiatric disorders, a much stronger signal is obtained when the 3D thresholded SPECT scan is performed rather than the conventional SPECT scan. Statistical parametric mapping is not required to economically produce images that can be easily read. These 3D thresholded SPECT images are shown in this study to have reasonable sensitivities and specificities for the detection of DSM-IV diagnosable ADHD.
1 Hipskind SG, Henderson TA, Mena I: Reply to APA Council on Children, Adolescents and Their Families on the use of NeuroSPECT in pediatric psychiatry. Alasbimn J 2005 7Google Scholar
2 : Scientifically unfounded claims in diagnosing and treating patients. Am J Psychiatry 2010; 167:598Crossref, Medline, Google Scholar
3 : Brain SPECT imaging in clinical practice. Am J Psychiatry 2010; 167:1125–1126, author reply 1125–1126Crossref, Medline, Google Scholar
4 : Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 1995; 2:148–156Crossref, Medline, Google Scholar
5 : Clinical investigation of cerebral blood flow, in Functional Radionuclide Imaging of the Brain. vol. 5. Edited by Magistretti PL. New York, Raven Press, 1983, pp 31–45Google Scholar
6 : Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci 1999; 354:1155–1163Crossref, Medline, Google Scholar
7 : Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling. Brain Res 2000; 886:108–112Crossref, Medline, Google Scholar
8 : Astrocytes as a predominant cellular site of 99mTc-HMPAO retention. Cereb Blood Flow Metab 2001; 21:456–468Crossref, Medline, Google Scholar
9 : High-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry 1997; 9:81–86Crossref, Medline, Google Scholar
10 : Review of two years of experiences with SPECT among psychiatric patients in a rural hospital setting. J Psychiatr Pract 2008; 14:318–323Crossref, Medline, Google Scholar
11 : Preliminary evidence differentiating ADHD using brain SPECT imaging in older patients. J Psychoactive Drugs 2008; 40:139–146Crossref, Medline, Google Scholar
12 : The value of HMPAO SPECT in predicting treatment response to citalopram in patients with major depression. Psychiatry Res 2009; 173:107–112Crossref, Medline, Google Scholar
13 : Does hypofrontality expand to global brain area in progression of schizophrenia?: a cross-sectional study between first-episode and chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:410–415Crossref, Medline, Google Scholar
14 Bonte FJ, Hynan L, Harris TS, et al: TC-99m HMPAO brain blood flow imaging in the dementias with histopathologic correlation in 73 patients. Int J Mol Imaging 2011; 2011:409101Google Scholar
15 : Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res 2011; 35:1771–1793Crossref, Medline, Google Scholar
16 : Brain perfusion in fibromyalgia patients and its differences between responders and poor responders to gabapentin. Arthritis Res Ther 2010; 12:R64Crossref, Medline, Google Scholar
17 : Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp 2005; 24:157–164Crossref, Medline, Google Scholar
18 : Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum Brain Mapp 2011; 32:601–611Crossref, Medline, Google Scholar
19 : Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naïve boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology 2011; 36:1575–1586Crossref, Medline, Google Scholar
20 : Brain SPECT imaging in complex psychiatric cases: an evidence-based, underutilized tool. Open Neuroimaging J 2011; 5:40–48Crossref, Medline, Google Scholar
21 Juni JE, Waxman AD, Devous MD, et al: Procedure Guideline for Brain Perfusion SPECT Using 99mTc Radiopharmaceuticals 3.0. J Nucl Med Technol 2009; 37:191–195Google Scholar
22 US Nuclear Regulatory Commission: Doses in Our Daily Lives. http://www.nrc.gov/about-nrc/radiation/around-us/doses-daily-lives.htmlGoogle Scholar
23 : Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007; 357:2277–2284Crossref, Medline, Google Scholar
24 : Images of Human Behavior: A Brain SPECT Atlas. Newport Beach, CA, Mindworks Press, 2004Google Scholar
25 : Predicting positive and negative treatment responses to stimulants with brain SPECT imaging. J Psychoactive Drugs 2008; 40:131–138Crossref, Medline, Google Scholar
26 : Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry 2012; 169:1038–1055Crossref, Medline, Google Scholar
27 : Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry 2006; 163:1052–1060Crossref, Medline, Google Scholar
28 : Intrasubject comparison between technetium-99m-ECD and technetium-99m-HMPAO in healthy human subjects. J Nucl Med 1992; 33:480–484Medline, Google Scholar
29 : Normal patterns on 99mTc-ECD brain SPECT scans in adults. J Nucl Med 2000; 41:1456–1464Medline, Google Scholar
30 Mena FJ, Mena I, Alamos BA, et al: SPECT Tc99m-HMPAO brain uptake in normal children: a comparison to normal elderly subjects. Alasbimn J 1998; 1. http://www.alasbimnjournal.cl/revistas/1/children_total.htmGoogle Scholar
31 Darcourt J, Mena I, Cauvin J-C, et al: Absolute calibration of HMPAO SPECT using 133-Xe rCBF values. Alasbimn J 1999; 2. http://www.alasbimnjournal.cl/revistas/5/darcourt1.htmGoogle Scholar
32 : Comparison of technetium-99m-HMPAO and xenon-133 measurements of regional cerebral blood flow by SPECT. J Nucl Med 1996; 37:1735–1740Medline, Google Scholar
33 : Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991; 11:690–699Crossref, Medline, Google Scholar
34 : The relationship between regional cerebral blood flow and response to methylphenidate in children with attention-deficit hyperactivity disorder: comparison between non-responders to methylphenidate and responders. J Psychiatr Res 2007; 41:459–465Crossref, Medline, Google Scholar
35 : Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Can J Psychiatry 2009; 54:651–664Crossref, Medline, Google Scholar
36 : Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR, 4th ed. Arlington, VA, American Psychiatric Publishing, 2000Google Scholar
37 : Specific ways brain SPECT imaging enhances clinical psychiatric practice. J Psychoactive Drugs 2012; 44:96–106Crossref, Medline, Google Scholar
38 First M, Botteron K, Carter C, et al: Consensus Report of the APA Work Group on Neuroimaging Markers of Psychiatric Disorders. Washington, DC, American Psychiatric Association, 2012Google Scholar
39 : Probabilistic Graphical Models. Cambridge, MA, MIT Press, 2009Google Scholar
40 : Accuracy of single-photon emission computed tomography in differentiating frontotemporal dementia from Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2007; 78:350–355Crossref, Medline, Google Scholar
41 : The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr 2012; 17:176–206Crossref, Medline, Google Scholar
42 : 99mTc-ECD brain perfusion SPECT in hyperalgesic fibromyalgia. Eur J Nucl Med Mol Imaging 2007; 34:130–134Crossref, Medline, Google Scholar
43 : Voxel-based statistical analysis of cerebral blood flow using Tc-99m ECD brain SPECT in patients with traumatic brain injury: group and individual analyses. Brain Inj 2006; 20:661–667Crossref, Medline, Google Scholar



