Maintenance Electroconvulsive Therapy for Aggression and Self-Injurious Behavior in Two Adolescents With Autism and Catatonia
Abstract
Frequent aggression toward others and repetitive self-injurious behaviors (SIB) can be features of catatonia in patients with autism. Similar to catatonia secondary to other etiologies, catatonia associated with autism responds well to treatment with benzodiazepines and/or electroconvulsive therapy (ECT). The authors report here on two adolescent patients with autism who presented with severe aggression, one of whom also engaged in repetitive SIB. With ongoing treatment with maintenance ECT, dramatic reduction in aggression and SIB were noted, allowing both patients a reasonable quality of life in their own homes. Attempts to taper off ECT coincided with return of aggression symptoms, although not SIB.
Catatonia is a neuropsychiatric syndrome of motor dysregulation, usually secondary to mood disorder, psychotic illness, or a general medical condition, which responds rapidly to treatment with benzodiazepines and/or electroconvulsive therapy (ECT).1,2 Over the past 2 decades, catatonia in patients with autism has been an increasingly recognized as a distinct subtype,3 and 12%–17% of adolescents and young adults with autism have been reported to have catatonic symptoms.4–6 Catatonia in patients with autism is often characterized with an increase in spontaneous aggression, repetitive self-injurious behaviors (SIB),7 echolalia, psychomotor retardation, increase in purposeless activity, unusual motor symptoms (posturing, mannerisms, stereotyped behaviors, or unusual gait), food refusal, and/or reduced speech or mutism. Some researchers have hypothesized that catatonia and autism may both be due to abnormalities in common neural circuits or neuropathological processes.8 It has also been hypothesized that autistic regression (loss of social skills after age 2 or 3 in children with autism) may represent a form of catatonic regression.9
A number of published case reports since 1999 have documented that ECT may successfully treat catatonia in patients with autism,3,10–12 including catatonia that presents with repetitive aggression and/or SIB.13–17 However, despite remission of symptoms after the acute phase of ECT, some of these patients experience a gradual return of aggression, SIB, and other symptoms of catatonia when ECT is either administered at reduced frequency or discontinued.15,16,18,19 This is not surprising, given that recurrence of symptoms is frequently noted in other conditions for which maintenance ECT is given, such as mood or psychotic disorders, if ECT is withheld.20 In 2010, Wachtel and colleagues published a case series of three autistic patients who required maintenance ECT for remission of catatonic symptoms after an initial course of ECT, with one patient having received a total of 286 treatments at the time of the publication.18
We report here on two adolescents with autism and catatonia who were treated with an index course of ECT, followed by maintenance ECT. Both patients had severe aggression toward others, and the second case had additionally experienced SIB that was refractory to medication or behavioral management. With maintenance ECT, both patients achieved a dramatic reduction in symptoms, allowing them to live at home, attend school, and have a reasonable quality of life. Attempts to taper off ECT were observed to result in return of symptoms. Both patients were treated for significant portions of their treatment course by either one or both of the authors. We obtained approval of the Institutional Ethics Board (IRB), and the cases are reported here using anonymous identifiers. Details regarding the number of episodes of aggression and SIB and their severity were meticulously kept in daily logs maintained by the parents; these were entered into the medical record at each ECT-related visit. These medical records were extensively reviewed and are described in this report.
Case Presentation #1
“CE” is a 16-year-old White boy with autism, common variable immune deficiency, and von Willebrand disease, who was admitted to the child and adolescent psychiatry unit at a midwestern university hospital for increasingly unpredictable episodes of spontaneously rushing at people and trying to bite and kick them, biting himself, throwing himself against walls, banging his head, and throwing objects across the room. CE had had such episodes since the age of 8, up to 15 times per day at the onset, but the frequency and unpredictability of these episodes had increased over several months before this admission. He also had occasional episodes of standing in a doorway for up to an hour on one foot and “freezing”. Before his admission, he had been tried on sertraline, risperidone, guanfacine, memantine, and topiramate, all of which had either increased his aggression or had not resulted in any significant benefit. Details about this patient’s initial ECT course have been reported in the past.21
Upon admission, CE was seen in 4-point restraints. He was minimally cooperative or communicative and had scratch marks on his face. He regurgitated food on his clothing, and then proceeded to lick it. When removed from restraints, he repeatedly hurled himself against the wall, yelling, “Another hospital!” During his first 4 weeks on the unit, CE displayed repeated, unpredictable episodes of agitation and SIB multiple times a day, during which he tried to hit and bite the staff, family members, or himself, or run into walls. There were no identifiable triggers to these behaviors, and diversions were of no benefit. He required slight physical contact from his mother to assist with motor tasks such as eating. He had frequent echolalia. He paced frequently, and his sleep was disrupted. A brain MRI and EEG were within normal limits. Routine laboratory investigations, including complete blood count, comprehensive metabolic panel, urinalysis, and thyroid function testing, were unremarkable. Metabolic testing, including pyruvate, carnitine profile, urine organic acids, and lactate, was nondiagnostic. There were no relevant findings in the extensive GI work-up that was performed to investigate repetitive food regurgitation. Unsuccessful medication trials to target the aggression included ziprasidone, lithium, and lamotrigine.
After more than a month into the admission, with no evidence of improvement with pharmacologic and behavioral management, CE’s case was brought to the attention of the ECT team, and CE was diagnosed with catatonia. All neuroleptics were discontinued; scheduled Ativan was started, and ECT was started at a 3-times-per-week schedule. All guidelines recommended by the American Academy of Child and Adolescent Psychiatry (AACAP) for the use of ECT in minors were followed.22 Medications administered during anesthesia for ECT were methohexital 80 mg for sedation, succinycholine as a muscle relaxant, glycopyrolate 0.2 mg as an antiarrhythmic, and flumazenil 0.5 mg to reverse the effect of benzodiazepines. Lorazepam 1 mg was administered on completion of ECT for its antidelirium action. ECT was administered with a MECTA spECTrum 5000Q machine, using bilateral technique. The initial treatment dose was 80 miliCoulombs (pulse width: 0.5 msec, frequency: 20 Hz, duration: 5.0 seconds, current: 0.8 A). The dose was gradually increased to the maximum of 576 mC by the 32nd treatment, which was continued for the remainder of the treatment course.
Treatment with ECT resulted in a gradual but dramatic improvement, which was noted in the frequency and intensity of aggression (see Figure 1). After his first 2–3 treatments, CE was noted to be markedly calmer, with an increasingly reactive affect. Unpredictable periods of aggression did continue, but the intensity and frequency were decreased. By Treatment #16, about 5 weeks after the first ECT, CE’s aggressive episodes had decreased significantly, and he was discharged home with the recommendation to continue with outpatient ECT. Over the course of the next 2 months, the ECT interval was gradually lengthened, until it was discontinued 4 months after discharge, after CE had received a total of 29 treatments. At this time, CE was having about 8 or 9 episodes of aggression per month, which were notably decreased in intensity and duration. He was able to attend school 4 days per week.
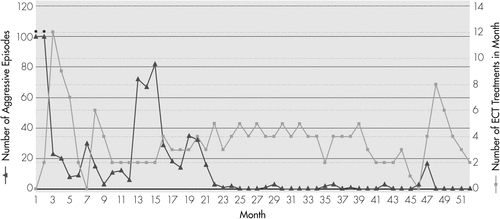
Month 1 indicates when the patient was first admitted to the hospital. An episode of aggression was defined as any incidence of spontaneously biting, hitting, kicking, punching, or throwing objects across the room.
aAsterisks represent data-points that are estimated because of lack of exact data.
Upon discontinuation of ECT, CE experienced a gradual decline in functioning, with an increase in frequency and intensity of aggressive behaviors, which progressively started to resemble the episodes at the pretreatment level. This necessitated reinitiation of ECT 2 months later, at a 3-day interval, which was tapered down to a 7-day interval over the next 2 months. This once again resulted in a decreased frequency of aggression, although CE continued to experience about 3–12 mild, brief episodes per month over the next 4 months (Months 9–12). Over the subsequent 3 months (Months 13–15), as ECT was further tapered down to an average 15-day interval, CE had an increased number of aggressive episodes, ranging from 67 to 82 episodes per month. His parents found it increasingly difficult to manage him at home because of the unpredictability and severity of his aggression. As a result, during Months 16–21, ECT interval was once again increased, back to every 7–10 days, with a resultant reduction in the number of aggressive episodes, which were reduced to approximately 14–35 per month.
Over the next 24 months (Months 22–45), CE did remarkably well. ECT was continued at an average 7-day interval, and CE went months at a time without any aggression. At Month 45, an EEG performed by neurology consultants captured seizure-like activity. No seizure activity had been noted during two previous EEG recordings. There was concern among some members of the team involved in CE’s care that seizures may be the primary contributors to his episodic agitation. At Month 46, after CE had been without aggressive behaviors for more than 1½ years, it was decided once again to attempt to discontinue ECT, based on the assumption that perhaps an aggressive antiepileptic regimen might be sufficient to control his symptoms. At that point, CE had received a total of 134 ECT treatments during his second treatment course. However, within 1 month of his last treatment, and despite receiving antiepileptic medications, CE began to show a return of aggressive behaviors, with 17 episodes of spontaneous aggression in 1 month. Episodes of “freezing” began to recur, and CE began to pace constantly, had frequent awakenings, and was no longer able to attend school. After 2 months of no response to titrating his antiepileptic medications to achieve therapeutic dosage (valproic acid, increased up to 1,000 mg daily, and zonisamide, up to 100 mg twice a day), ECT was restarted, with an immediate cessation of aggressive behaviors. During the subsequent 5 months (Months 48–52), ECT interval has been gradually increased from every 3 days to every 15 days, with no return of aggressive behaviors to date. At the time of writing this report, CE has received a total of 31 treatments during this third treatment course, and a total of 181 treatments over three courses spanning 51 months.
Several medications were tried during CE’s treatment course in an attempt achieve longer intervals between ECT treatments and to eventually taper off ECT. These included lorazepam, phenytoin, riluzole, carbamazepine, oxcarbazepine, valproic acid, and zonisamide (see Table 1). None of the many anticonvulsants that were tried during CE’s treatment course resulted in improvement of symptoms. It should be noted that the initiation of riluzole did coincide with the 2-year period during which CE was almost symptom-free. However, he was also receiving weekly ECT during this time. At the time of writing, in addition to ECT administered at twice-per-month intervals, CE continues to receive valproic acid 750 mg daily, lorazepam 22 mg daily, and riluzole 100 mg daily. He has remained free of aggression and SIB for 5 months.
| Medication | Daily Dose Range | Months Tried | Outcome |
|---|---|---|---|
| Lorazepam | 5–14 mg | 2–15 | Unclear relationship with symptoms |
| Lorazepam | 20–24 mg | 16–present | Attempts to taper resulted in worsening of aggression |
| Riluzole | 25–100 mg | 24–present | Some benefit in aggression |
| Phenytoin | 25–100 mg | 31–36 | Lack of clear benefit, possible worsening of aggression |
| Carbamazepine | 400 mg | 40–41 | Discontinued because of thrombocytopenia |
| Oxcarbazepine | 600 mg | 42 | Discontinued because of worsening aggression |
| Valproic acid | 500–1,000 mg | 41–present | Unclear benefit on aggression |
| Zonisamide | 50–200 mg | 45–47, 49–50 | Discontinued because of worsening aggression |
Case Presentation #2
“DS” is a 15-year-old, African American, girl, with autism, macrocephaly, polycystic ovarian syndrome, and moderate cognitive impairment, who was admitted to the inpatient unit of a midwestern hospital for increasing frequency of spontaneous episodes of punching, kicking, and biting, often requiring her to be restrained by several adults. These episodes occurred about 4–5 times per day, lasted 10–20 minutes, and resolved spontaneously. After these episodes, DS behaved as if nothing had happened. There was no obvious trigger, and the violence was indiscriminately directed toward parents, teachers, or peers. It was noteworthy that DS did not display any SIB.
DS was born at term as a product of a normal pregnancy. She was largely nonverbal, communicating occasionally with one or two words. She did reasonably well until age 11, enjoying home and school life, until the start of the aggressive episodes. By the time she came to our attention, she had been hospitalized on eight different occasions. Previous medication trials had included several antiepileptic agents (valproic acid, topiramate, oxcarbazepine, lamotrigine), antipsychotics (risperidone, aripiprazole, quetiapine, ziprasidone, clozapine, chlorpromazine, and haloperidol), alpha-2 agonists (clonidine and guanfacine), stimulants (methylphenidate and dextroamphetamine), lithium, and SSRIs (sertraline, paroxetine), all with either no benefit or worsening of symptoms. Behavioral treatment, including attempts to identify and address potential triggers or secondary gains, was unsuccessful.
Upon admission, DS was noted to be a tall, heavy-set girl with an intense stare. She was largely nonverbal, except for notable echolalia and occasional simple vocalizations to communicate her needs (e.g., “Mom”). She displayed multiple, unpredictable episodes of aggression on a daily basis, injuring staff members on two different occasions. She required constant supervision by two staff members, and restraints had to be applied almost daily to ensure safety of the patient and staff. An extensive laboratory work-up, including thyroid-function testing, complete blood count, comprehensive metabolic panel, urinalysis, and toxicology screen, was unrevealing. A brain MRI was unremarkable, except for a 2-cm vascular malformation in the left cerebrum, which was deemed as being non-significant by the neurosurgery consult team. Her medications on admission included clozapine and lithium, which were increased to 300 mg at bedtime and 450 mg three times per day, respectively. Additional changes included initiation of clonazepam 3 mg at bedtime, lorazepam up to 2 mg four times per day, and trazodone up to 200 mg at bedtime (all were slowly titrated up). However, none of these medication changes had any significant benefit, and DS was discharged after 1 month with minimal improvement in symptoms.
DS was readmitted 2 weeks after discharge for ongoing severe aggression because her parents were not able to manage her at home. On the inpatient unit, she continued to display unprovoked episodes of aggression multiple times per day. Early during this second admission, the case was brought to the attention of our ECT team, and a diagnosis of agitated catatonia was made based on symptoms of severe agitation, frequent and unprovoked excitement, and stereotyped behaviors (repeatedly changing clothes, repeated turning of head to one side with mumbling movements of the lips). Using the AACAP guidelines for ECT in minors, including consultation with two additional pediatric psychiatrists, we started ECT. Medications used for anesthesia during the treatment included methohexital 100 mg for sedation, succinylcholine 100 mg for muscle relaxation, and glycopyrolate 0.2 mg to prevent arrhythmias. Also, flumazenil 1 mg was administered before ECT to reverse the effect of benzodiazepines, and midazolam 2 mg and lorazepam 2 mg were administered after ECT to prevent delirium. ECT was administered with a MECTA spECTrum 5000Q machine, using bilateral electrode placement. The initial treatment dose was 48 miliCoulombs (pulse width: 0.5 msec, frequency: 20 Hz, duration: 3 sec, current: 0.8 A). The dosage was gradually increased to the maximum dose of 576 mC by Treatment 16, which was continued for the remainder of her treatment course.
Administration of the first ECT treatment resulted in significantly increased agitation and decreased sleep, which was attributed to manic symptoms precipitated by ECT. Given the severity of her symptoms and potential for injury, ECT frequency was increased to once-daily treatments for the next 3 days, with minimal improvement, and then two seizures per day for the next 2 days. This “en bloc” treatment strategy has been used when urgent response is necessary because of high symptom severity.23,24 This strategy resulted in significant decrease in agitated behaviors (see Figure 2). ECT frequency was subsequently decreased to 3 times per week. DS remained hospitalized for 5 weeks, receiving a total of 17 treatments. At the time of her discharge, she had been free of aggressive behaviors for 6 days. The recommendation at discharge was that ECT should be continued on an outpatient basis, and the frequency of treatment should be adjusted on the basis of clinical assessment (individualized maintenance treatment).
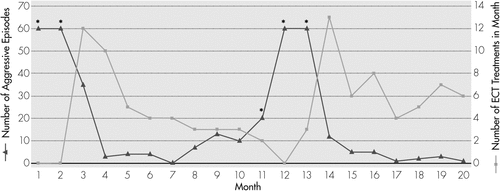
Month 1 indicates when the patient was first admitted to the hospital. An episode of aggression was defined as any incidence of spontaneously biting, hitting, kicking, punching, or throwing objects across the room.
aAsterisks represent data-points that are estimated because of lack of exact data.
Over the next 5 months, ECT was continued at about approximately a 6- to 8-day interval. During this time, DS’s behaviors remained markedly improved. She had an average of three episodes of aggression per month, which was a markedly decreased frequency. Her family described her improvement with ECT as “a miracle.” However, as the ECT interval was lengthened to 10 days, during Months 6–7, DS had a slight increase in frequency of agitated/ aggressive behaviors, with an average of 10 incidents per month. There was increasing pressure from DS’s school to consider out-of-home placement because of her aggressive episodes in school. At Month 11, because of this increase in aggressive episodes, DS was admitted to an outside hospital for 1 week, where lorazepam, lamotrigine, and trazodone, were discontinued, and guanfacine was started. By the time she was discharged, DS was severely dysregulated, with significant increase in frequency and intensity of aggressive episodes. At this point, ECT had to be discontinued because her parents could no longer transport DS for treatment because of worsening behaviors. Over the next 2 months, as ECT treatments were withheld, DS’s aggressive episodes returned to the full pre-ECT intensity, and she was experiencing up to more than 60 episodes each month. DS was readmitted to the child and adolescent psychiatry unit at Month 13 to restart ECT. With reinitiation of ECT, episodes of violent agitation decreased dramatically in number, down to 12 episodes in Month 14, 5 episodes during Month 15, and only 1 during Month 17. ECT has continued since then, with an average 5-day interval, with markedly good control of aggression since. To date, DS has received a total of 105 ECT treatments, 48 during the first course, and 57 during the second, over a period of 20 months.
Several medications have been tried during the course of DS’s treatment to target aggression and allow for longer ECT intervals (Table 2). Similar to CE, none of these medications have resulted in change in aggression that could be considered independent of ECT. At the time of writing of this report, DS’s medication regimen includes lorazepam 4 mg, 4 times per day, oxcarbazepine 600 mg twice daily, lithium 1,050 mg daily, and trazodone 100 mg at bedtime. Also, she continues to receive ECT treatments every 5 days.
| Medication | Daily Dose Range | Months Tried | Outcome |
|---|---|---|---|
| Lithium | 1,050–1,350 mg | 1–present | Unclear relationship with symptoms |
| Clonazepam | 3 mg | 1 | No improvement in symptoms |
| Lorazepam | 12–16 mg | 1–present | Unclear correlation with symptoms |
| Trazodone | 100–200 mg | 1–present | Unclear correlation with symptoms |
| Clozapine | 300 mg | 1–2 | No improvement in aggression |
| Haloperidol | 2 mg | 1–2 | No improvement in aggression |
| Chlorpromazine | 100 mg | 3–12 | Unclear correlation with symptoms |
| Lamotrigine | 25 mg | 10–11 | Correlated with worsening aggression |
| Guanfacine | 2 mg | 12–13 | Correlated with worsening aggression |
| Oxcarbazepine | 150–1,200 mg | 13–present | Correlated with improvement in symptoms |
Discussion
Not all “challenging” or aggressive behaviors in patients with autism are symptomatic of catatonia. Sometimes, patients with autism engage in destructive behaviors in order to achieve a secondary gain or as part of a general pattern of negative behavior. Such behaviors can be identified by careful behavioral analysis and should be treated accordingly. However, when aggressive symptoms or SIB are repetitive, occur with high frequency, are a change from baseline functioning, co-occur with other symptoms of catatonia, and there is no secondary gain, the clinician should have a high suspicion for the diagnosis of catatonia.
The cases described here raise important questions with regard to what might constitute optimum treatment for “CE,” “DS,” and other similar cases seen in clinical practice. For instance, for how long should maintenance ECT be continued? Is it appropriate, or even ethical, to withdraw maintenance ECT from such patients when it allows them to function at a level that is closely consistent with their premorbid level of functioning? Is there a rationale to repeat trials of psychotropic agents that have been unhelpful in the past and may carry potentially serious side effects?
There is little in the literature on these topics to guide treatment. Therefore, at this time, until more systematic studies can be conducted, we may only rely on what appears to be empirically effective for our patients. Maintenance ECT has enabled CE and DS to live at home with loving and devoted parents, attend school with peers, and enjoy a reasonable quality of life. Without maintenance ECT, both patients would almost certainly require out-of-home placement, as is often the case in children with a diagnosis of autism who have behavioral problems involving severe assault with high risk to the caregivers.25 Moreover, without maintenance ECT, their ability to participate in educational programs with their peers or to reach their full potential would be compromised. They would be at a higher risk for medical complications: Other patients with similar presentations have been reported to suffer from traumatic cataracts requiring surgery,13 head injuries,14 broken limbs,16 skin bruises/excoriations/keloidal scars,15 and chronic cellulitis from repeated skin-biting.16 DS and CE have tolerated ECT well, with no physical side effects such as prolonged seizures or nausea/vomiting that has persisted beyond the immediate post-ECT recovery period. From a neuropsychological perspective, they continue to function at their respective baseline level, per parent report, and their functioning appears to be consistent with their moderate-or-severe mental retardation. Attempts to withdraw ECT have resulted in return of spontaneous aggression, with notably decreased functioning. Furthermore, there has been minimal benefit from medication trials, including maximum doses, or from behavioral management.
For the purpose of monitoring any patient with autism and catatonia who is receiving maintenance ECT, we recommend at least monthly visits for assessment of the number and intensity of aggressive episodes and SIB, potential for injury, additional symptoms of catatonia (grimacing, posturing, freezing), food and fluid intake, affective symptoms, and overall level of impairment. As can be noted in Figure 1 and Figure 2, an optimal maintenance ECT schedule for patients CE and DS appears to be about 1 treatment per week. However, for other patients undergoing maintenance ECT, it is best to arrive at an individualized ECT treatment schedule, based on symptom severity. The consideration to use formal neuropsychological testing before ECT and during the maintenance phase should be decided on a case-by-case basis. For CE and DS, pre-ECT neuropsychological testing was not completed because of their level of impairment. Instead, ongoing cognitive assessment was based on monitoring their academic performance and ability to participate in activities of daily living.
Many psychiatrists are known to express discomfort, fear disapproval, or anticipate legal problems at the prospect of using ECT in a child with mental retardation or developmental disorders.26 However, ECT has been repeatedly shown to be a well-tolerated treatment, in both children and adults, with minimal risks. No mortality has been reported from ECT in children and adolescents. The most common side effects of ECT in children include transient headaches (15% of patients), transient delirium lasting less than 1 hour (5%), agitation (3%), hypomanic symptoms (2%), subjective memory loss (2%), and vomiting (1%).27 Several studies (albeit retrospective) on the effects of ECT on cognition in children and adolescents have shown no evidence of cognitive decline in children who have been treated with ECT, in both short- and longer-term follow-up.28–30 A recent case report by Wachtel and colleagues demonstrated no decline in performance on neuropsychological testing of a young man with autism who received maintenance ECT for 2 years.19 The reluctance to use ECT in children or in youth with developmental disabilities appears to be more likely related to a lack of knowledge and experience. For instance, in a 2001 survey of child psychiatrists and psychologists, 54% of respondents stated that they had minimal knowledge about the use of ECT in children, whereas those with more advanced knowledge reported a higher perception of safety and efficacy.31 Clearly, negative perception of ECT, an exaggerated sense of danger to the child when considering ECT, and lack of adequate knowledge about ECT among child and adolescent psychiatrists are closely-associated factors and deserve to be addressed systematically.
Conclusion
Maintenance ECT is commonly used in the treatment of many psychiatric conditions in the adult population. Similarly, maintenance-ECT has been found to be effective in adolescents with depression when medications alone are insufficient to maintain euthymia.32 Based on our experience, withdrawal of maintenance-ECT in patients with autism and catatonia often precipitates relapse of symptoms, perhaps more rapidly and predictably than in the treatment of mood disorders. We can only hypothesize that some neuronal dysregulation, perhaps at a neurotransmitter level, is responsible for these symptoms, which are improved by ECT. Irrespective of the exact mechanism of action, it is clear that ongoing treatment is essential for sustained improvement. In such cases, we propose that maintenance-ECT should be continued for as long as there is clear benefit to the patient. Further research is necessary to identify ideal parameters for maintenance-ECT in this population. Investigation into the effectiveness of unilateral ECT technique or transcranial magnetic stimulation (TMS) in the treatment of these symptoms is also warranted. Above all, ongoing advocacy and educational efforts about this potentially life-saving treatment modality are important.
1 : Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003; 160:1233–1241Crossref, Medline, Google Scholar
2 : Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep 2010; 12:180–185Crossref, Medline, Google Scholar
3 : Catatonia in autism: a distinct subtype? J Intellect Disabil Res 2005; 49:102–105Crossref, Medline, Google Scholar
4 : Catatonia in autistic spectrum disorders. Br J Psychiatry 2000; 176:357–362Crossref, Medline, Google Scholar
5 : Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord 2005; 35:351–360Crossref, Medline, Google Scholar
6 : Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand 2012; 125:33–38Crossref, Medline, Google Scholar
7 : Self-injury in autism as an alternate sign of catatonia: implications for electroconvulsive therapy. Med Hypotheses 2010; 75:111–114Crossref, Medline, Google Scholar
8 : Is there a common neuronal basis for autism and catatonia? Int Rev Neurobiol 2006; 72:151–164Crossref, Medline, Google Scholar
9 : Are autistic and catatonic regression related? a few working hypotheses involving GABA, Purkinje cell survival, neurogenesis, and ECT. Int Rev Neurobiol 2006; 72:55–79Crossref, Medline, Google Scholar
10 : Catatonia, autism, and ECT. Dev Med Child Neurol 1999; 41:843–845Crossref, Medline, Google Scholar
11 : Catatonia in autistic spectrum disorders: a medical treatment algorithm. Int Rev Neurobiol 2006; 72:233–244Crossref, Medline, Google Scholar
12 : Catatonia in autistic twins: role of electroconvulsive therapy. J ECT 2007; 23:21–22Crossref, Medline, Google Scholar
13 : ECT for catatonia in an autistic girl. Am J Psychiatry 2008; 165:329–333Crossref, Medline, Google Scholar
14 : ECT for self-injury in an autistic boy. Eur Child Adolesc Psychiatry 2009; 18:458–463Crossref, Medline, Google Scholar
15 : Electroconvulsive therapy in a man with autism experiencing severe depression, catatonia, and self-injury. J ECT 2010; 26:70–73Crossref, Medline, Google Scholar
16 : Electroconvulsive therapy for psychotropic-refractory bipolar affective disorder and severe self-injury and aggression in an 11-year-old autistic boy. Eur Child Adolesc Psychiatry 2011; 20:147–152Crossref, Medline, Google Scholar
17 : Electroconvulsive therapy in adolescents with intellectual disability and severe self-injurious behavior and aggression: a retrospective study. Eur Child Adolesc Psychiatry 2013; 22:55–62Crossref, Medline, Google Scholar
18 : Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:581–587Crossref, Medline, Google Scholar
19 : Stability of neuropsychological testing during two years of maintenance electroconvulsive therapy in an autistic man. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:301–302Crossref, Medline, Google Scholar
20 : One-year follow-up after discontinuing maintenance electroconvulsive therapy. J ECT 2012; 28:225–228Crossref, Medline, Google Scholar
21 : Onset of catatonia at puberty: electroconvulsive therapy response in two autistic adolescents. J ECT 2010; 26:274–277Crossref, Medline, Google Scholar
22 ;
23 : Multiple ECT late in the course of neuroleptic malignant syndrome. Convuls Ther 1997; 13:269–273Medline, Google Scholar
24 : Electroconvulsive therapy for catatonia. Am J Psychiatry 2010; 167:1127–1128, author reply 1128Crossref, Medline, Google Scholar
25 : Predictors of Out-of-Home Placement Among Children with Developmental Disabilities. Olympia, WA, Washington State Dept. of Social and Health Services, Research and Data Analysis Division, 2008Google Scholar
26 : Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford, Oxford University Press, 2009Google Scholar
27 : Electroconvulsive therapy in treatment-resistant bipolar youth. J Child Adolesc Psychopharmacol 1995; 5:167–175Crossref, Google Scholar
28 : Electroconvulsive treatment in adolescents with pharmacotherapy-refractory depression. J Child Adolesc Psychopharmacol 1996; 6:259–271Crossref, Medline, Google Scholar
29 : Absence of cognitive impairment at long-term follow-up in adolescents treated with ECT for severe mood disorder. Am J Psychiatry 2000; 157:460–462Crossref, Medline, Google Scholar
30 : Cognitive side effects of electroconvulsive therapy in adolescents. J Child Adolesc Psychopharmacol 2000; 10:269–276Crossref, Medline, Google Scholar
31 : Electroconvulsive therapy for minors: experiences and attitudes of child psychiatrists and psychologists. J ECT 2001; 17:109–117Crossref, Medline, Google Scholar
32 : Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT 2011; 27:168–174Crossref, Medline, Google Scholar



