Association of BDNF Val66MET Polymorphism With Parkinson’s Disease and Depression and Anxiety Symptoms
Abstract
An association between Parkinson’s disease (PD) and brain-derived neurotrophic factor (BDNF) was suggested by several studies, with contradictory results. BDNF is necessary for the survival of dopaminergic neurons in substantia nigra. Val66Met is a common polymorphism of the BDNF gene that affects cognitive and motor processes. The authors studied 104 Brazilian patients with PD and 96 control participants. The G/G genotype was significantly associated with depression and anxiety symptoms and development of PD. This is the first study that associates this genotype with PD.
Parkinson’s disease (PD) is a progressive neurodegenerative disease that affects mainly dopaminergic neurons of the substantia nigra pars compacta (SNpc), leading to motor symptoms (tremor, bradykinesia, rigidity, and postural instability). PD also presents nonmotor symptoms, such as cognitive impairments and emotional disturbances (depression and anxiety). Although the direct cause is unknown, PD is a multifactorial disease influenced by environmental and genetic factors, with aging being the core predisposing factor.1
Brain-derived neurotrophic factor (BDNF) is widely expressed in the central nervous system. This protein binds to tropomyosin-related kinase B (TrkB) receptor to promote neuronal growth, survival, differentiation, and synaptic plasticity.2,3 Studies have proposed that BDNF has an important role in PD. BDNF is necessary for the establishment of dopaminergic neurons in the SNpc, and 70% of these neurons express BDNF protein and TrkB.2,3 Furthermore, BDNF protects dopaminergic neurons against neurotoxin-induced neuronal death in animal models of PD.4 Therefore, failure or decrease in neurotrophy supported by BDNF could be an etiologic factor for PD.1
Studies have investigated a relationship between the development of PD and the presence of a common polymorphism in the BDNF gene, Val66Met (rs6265). BDNF Val66Met is a single nucleotide polymorphism (G to A) that results in the substitution of valine (Val) for methionine (Met) at position 66 in the prodomain region. This alteration decreases the interaction of the precursor form of BDNF (pro-BDNF) with sortilin (which mediates the transport of pro-BDNF). This leads to decrease in BDNF dendritic distribution, reduced BDNF transport to secretory granules, and impairment of the activity-dependent secretory pathway of BDNF.2,5 Several studies investigated a relationship between the Met variant of BDNF Val66Met polymorphism and depression, anxiety, and cognitive performance in healthy individuals.6,7 Also, a positive association with cognitive impairment,8 planning ability9 and early development of dyskinesia10 was observed in PD patients.
Nevertheless, only one case-control study observed homozygosity of A allele for the BDNF Val66Met polymorphism as a risk factor for PD, in a Japanese population.11 Other studies failed to report such association12,13 and even described the opposite association (G allele more frequent in PD).14 Association studies of this polymorphism are relevant because frequency and effects of the polymorphism can be different between distinct populations.7 In Brazil, an effect of this polymorphism on cognition in PD patients has been observed.15 However, other analyses, such as genetic frequency comparisons with control individuals, or evaluations of other clinical features were not performed. The present study investigates this polymorphism in control subjects and PD patients in a Brazilian population. We hypothesized that BDNF polymorphism Val66Met is associated with PD and that this association is relevant to specific symptoms of the disease, namely anxiety and depression, given previous studies that associated BDNF polymorphisms with these disorders in non-PD individuals.2,16,17
Patients and Methods
PD patients (N=104) were recruited from Onofre Lopes University Hospital in Natal, Rio Grande do Norte (Brazil). Diagnosis of PD was based on the United Kingdom Parkinson’s Disease Society Brain Bank diagnostic criteria18 and confirmed by a certified neurologist specialized in PD. Control subjects (N=96) with no kinship with patients and no history of neurological diseases were recruited in the hospital. The groups were matched by age and sex. This study was approved by the Ethics Research Committee of the Onofre Lopes University Hospital. All participants signed the written informed consent.
The BDNF Val66Met polymorphism was genotyped by polymerase chain reaction amplification and restriction enzyme digestion.17 Genomic DNA was extracted from EDTA-containing peripheral blood samples (commercial kit FlexiGene DNA kit, QIAGEN, Germany). DNA integrity was assessed on a 1% ethidium bromide stained agarose gel, and quantitation was performed in SpectraMax 190 spectrophotometer ultraviolet-Vis Microplate Reader (Molecular Devices, United States). The following primers were used: BDNF Forward 5′-AAA GAA GCA AAC ATC CGA GGA CAA G-3′ and BDNF Reverse 5′-ATT CCT CCA GCA GAA AGA GAA GAG G-3′, which resulted in a 274 base pair (bp) PCR product. The thermocycler Esco’s Swift Maxi Thermal Cycle (United States) was used for DNA amplification. Amplification reactions were performed in a total volume of 25μL (5pmol of each primer, 200μM deoxyribonucleotide triphosphate (dNTP), 1×NHSO4 buffer, 1.5mM MgCl2, 0.75U Taq DNA polymerase; Fermentas-Thermo Fisher Scientific, United Kingdom) and 2 μl of pure DNA sample. The PCR cycling conditions were an initial denaturation for 3 minutes at 95°C, 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 2 minutes, and a final extension at 72°C for 5 minutes. The BDNF V66M polymorphism was differentiated using the NlaIII (10 units/μl) (Uniscience-Bio Labs, New England, Ipswich, Mass.) restriction enzyme. Amplicons and restriction fragments were visualized after electrophoresis on a 3% ethidium bromide agarose gel (Figure 1).
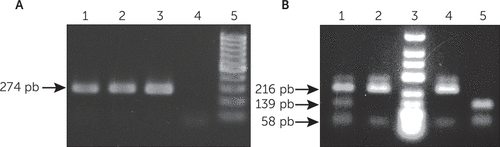
FIGURE 1. Products of PCR Amplificationa
a A) Standard band after PCR amplification of the region containing the BDNF Val66Met polymorphism (row 1–3), negative control (row 4) and GeneRulerTM 100 base pair (bp) DNA ladder marker (row 5); B) BDNF Val66Met polymorphism genotyping using the restriction enzyme NlaIII. After PCR product digestion (274 bp) homozygous for G allele presented two bands of 216 bp and 58 bp (row 2 and 4), homozygotes for A allele showed two bands of 139 bp and 58 bp (row 5) and heterozygotes (A/G) showed all three bands of 216, 139 and 58 bp (row 1). Row 3 is Thermo Scientific GeneRulerTM Low Range marker for identification of the bands.
The following clinical evaluations were performed: age at PD onset, first symptom, side of symptom initiation, disease progression, Unified Parkinson´s Disease Rating Scale (UPDRS), Hoehn & Yahr (HY) scale, Schwab & England Activities Daily Living Scale (SE), Beck Depression Inventory (BDI), and Beck Anxiety Inventory (BAI).
The data were presented as mean±standard deviation [minimum value; maximum value]. Clinical outcomes between groups were compared with Mann-Whitney test given the nonparametric nature of the data. Chi-square test was used to compare the allele and genotype frequencies between control and PD patients. Adjusted residues with corrected Bonferroni p values were used as post hoc analysis.19 The analyses of the Hardy-Weinberg Equilibrium (HWE) and polymorphism association with PD were performed in the SNPstats program.20 Binary logistic regression model was used to estimate the effect of genotype in association with other factors on the probability of development of specific symptoms of PD. Power analysis was performed using the G*Power statistical program.21 The analysis was considered significant when p<0.05.
Results
The 104 patients (73 men and 31 women) recruited for the PD group presented a mean age of 64.32±11.71 [37–89], mean age at onset of 55.70±11.97 [30–87], and 8.76±5.79 [1–32] years of progression of the disease. The 96 subjects (67 men and 29 women) of the control group presented a mean age of 63.15±10.18 [38–88]. No significant difference in age was observed between groups [U=4434.000, p=0.359]. Thirty-seven percent (37%) of PD patients had early Parkinson’s onset (EPDO; i.e., first symptoms before or at 50 years old), 44% reported positive family history, and 17.3% presented both conditions. Moreover, 70% described their first symptom as involuntary tremors, and 56% exhibited the disease as unilateral at first. Across the progression of the disease, all patients presented bilateral manifestations eventually.
Distribution of allele and genotype frequency was in agreement with HWE (p>0.05). Among PD patients, 75 (72.1%) had G/G genotype, while 26 (25%) had one copy and three (2.9%) had two copies of the BDNF variant allele A. The BDNF Val66Met genotype frequencies observed were similar to those previously seen in Brazilian PD patients.16 In the control group, the frequency of homozygotes was 50 (52.1%) for the G allele, two (2.1%) for the A allele, and 44 (45.8%) were heterozygotes. The genotypes showed a statistically different frequency between the control group and PD patients (χ2(2)=9.524, p=0.009), with the G/G (Bonferroni-adjusted residue, p<0.001) and A/G (Bonferroni-adjusted residue, p<0.001) genotypes being significantly more frequent in the PD and control groups, respectively (corrected significant, p=0.0083). Allele frequencies were not significantly different between the groups (χ2(1)=3.712, p=0.054). Genotypes and allele frequencies are presented in Table 1.
| Allele Distributiona | Genotype Distributionb | ||||
|---|---|---|---|---|---|
| Allele | Control Group | PD Group | Genotype | Control Group | PD Group |
| G | 143 (74%) | 176 (85%) | G/G | 50 (52.1%) | 75 (72.1%) |
| A | 49 (26%) | 32 (15%) | A/G | 44 (45.8%) | 26 (25%) |
| A/A | 2 (2.1%) | 3 (2.9%) | |||
TABLE 1. Distribution of Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism Alleles and Genotypes in Patients With Parkinson’s Disease (PD) (N=104) and in Control Subjects (N=96) in a Brazilian Population
In order to perform binary logistic regression analysis, we pooled cases with A/G and A/A together due the small number of individuals with A/A genotype. The association analysis indicated that the A allele presented a protective factor against PD with an odds ratio (OR) equal to 0.42 (95% CI=0.22–0.72, p=0.003; Table 2). Power analysis showed that this regression had 81.2% power to detect an association.
| Allele | Frequency N (%) | Analysis | |||
|---|---|---|---|---|---|
| Control | Parkinson’s Disease | Odds Ratio | 95% CI | p | |
| G/G | 50 (52.1%) | 75 (72.1%) | 1.00 | 0.004* | |
| A/G+A/A | 46 (47.9%) | 29 (27.9%) | 0.42 | 0.23–0.76 | |
TABLE 2. Frequency of Presence of A Allele Versus Absence of A Allele in Brain-Derived Neurotrophic Factor Val66Met Polymorphism Between Groups and Association Analysis (Binary Logistic Regression) of this Genetic Contrast (G/G Versus A/G+A/A) Using Group (Control or Parkinson’s Disease Patients) as the Response Variable
PD patients presented a significant reduction in functional independence (97.70±6.49 [60;100] versus 64.83±21.44 [20;90]) as measured in SE [U=296.000, p<0.001], higher UPDRS scores (4.58±5.84 [0;34] versus 45.11±19.82 [12;95]) [U=101.000, p<0.001], and more presence and severity of anxiety and depression symptoms as evaluated by BAI (9.35±8.63 [0;37] versus 16.52±9.51 [0;47]) and BDI (7.18±7.80 [0;47] versus 16.22±9.52 [2;47]) [U=2548.000, p<0.0001; 1857.500, p<0.0001], respectively, when compared with controls.
Table 3 shows the scores obtained in clinical symptoms evaluation in PD group according to genotype. There were no significant differences between the investigated genotypes. In addition, none of the clinical parameters evaluated were statistically different between carries and noncarriers of A allele in control group.
| Variable | BDNF Val66Met Genotype | p | Total | |
|---|---|---|---|---|
| G/G | A/G+A/A | |||
| Age at PD onset | 54.69±11.1 [30;77] | 58.28±13.6 [35;87] | 0.180 | 55.70±11.9 [30;87] |
| Unified Parkinson’s Disease Rating Scale | ||||
| I | 1.08±0.94 [0;3] | 1.07±0.97 [0;4] | 0.764 | 1.08±0.94 [0;4] |
| II | 19.77±8.89 [3;37] | 18.46±8.22 [4;31] | 0.942 | 19.41±8.6 [3;37] |
| III | 21.72±10.8 [5;47] | 21.07±8.07 [9;37] | 0.878 | 21.54±10.0 [5;47] |
| Total | 46.11±21.07 [12;95] | 43.53±16.7 [17;75] | 0.975 | 45.40±19.9 [12;95] |
| Hoehn and Yahr | 2.50±1.16 [1;4] | 2.59±1.10 [1;5] | 0.751 | 2.52±1.08 [1;5] |
| Schwab and England Activities Daily Living Scale | 64.41±20.9 [20;90] | 66.15±22.1 [30;90] | 0.823 | 64.89±21.1 [20;90] |
| Beck Depression Inventory | 16.92±9.12 [2;47] | 13.88±9.30 [3;41] | 0.073 | 16.08±9.2 [2;47] |
| Beck Anxiety Inventory | 17.30±9.52 [0;40] | 15.07±10.44 [0;47] | 0.246 | 16.69±9.7 [0;47] |
TABLE 3. Nonsignificant Distribution of Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism by Presence of A Allele in Patients With Parkinson’s Disease (PD) in a Brazilian Population in Relation to Clinical Parametersa
We also performed binary logistic regression analyses to verify if the BDNF genotype could exert an influence on development of depression and anxiety in PD. In association with other factors (Table 4, Table 5), PD group with G/G genotype presented a threefold risk (OR=3.4; IC 95%: 1.02–11.39; p=0.04) for depression and a fourfold risk for anxiety (OR=4.0; IC 95%: 1.12–14.94; p=0.03). Post hoc analysis showed a 99% power for both regressions, indicating that the size sample was adequate to analysis. This effect was only observed in the PD group. In the control group, the G/G genotype or the A allele did not influence the development of depression (G/G genotype: OR=1.139; IC 95%: 0.403–3.217; p=0.806; A allele: OR=0.878; IC 95%: 0.311–2.481; p=0.806) or anxiety (G/G genotype: OR=0.857; IC 95%: 0.304–2.416; p=0.771; A allele: OR=1.166; IC 95%: 0.414–3.286; p=0.771).
| Covariate | Estimate | Standard Error | Wald Chi-Square | p | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Sex | –0.116 | 0.650 | 0.032 | 0.858 | 0.890 | 0.249–3.183 |
| Progression | 0.171 | 0.077 | 4.954 | 0.026a | 1.186 | 1.021–1.378 |
| G/G BDNF Val66Met | 1.411 | 0.660 | 4.567 | 0.033a | 4.099 | 1.124–14.949 |
| Positive family history | 1.549 | 0.646 | 5.751 | 0.016a | 4.709 | 1.327–16.708 |
| Unified Parkinson’s Disease Rating Scale Ib | 1.026 | 0.377 | 7.420 | 0.006a | 2.790 | 1.333–5.839 |
| Constant | –2.667 | 1.042 | 6.558 | 0.010a | 0.069 | — |
TABLE 4. Multivariate Analysis of Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism for Development of Anxiety in Parkinson’s Disease Patients by Fitting Multiple Logistic Regression Model
| Covariate | Estimate | Standard Error | Wald Chi-Square | p | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Sex | –0.714 | 0.650 | 1.205 | 0.272 | 0.490 | 0.137–1.752 |
| Progression | 0.041 | 0.071 | 0.339 | 0.560 | 1.042 | 0.907–1.197 |
| G/G BDNF Val66Met | 1.227 | 0.615 | 3.980 | 0.046a | 3.412 | 1.022–11.391 |
| Unified Parkinson’s Disease Rating Scale total | 0.078 | 0.022 | 12.414 | 0.000a | 1.081 | 1.035–1.128 |
| Constant | –2.608 | 0.982 | 7.051 | 0.008a | 0.074 | — |
TABLE 5. Multivariate Analysis of Brain-Derived Neurotrophic Factor (BDNF) Val66Met Polymorphism for Development of Depression in Parkinson’s Disease Patients by Fitting Multiple Logistic Regression Model
Discussion
We investigated the possible association of the BDNF Val66Met polymorphism with the development of PD in a Brazilian sample. Many studies have investigated this association, with contradictory overall results. One study observed a higher frequency of homozygosity (A/A) in PD patients in a Japanese population, but the control group was not in accordance with HWE.11 Other studies found no association.12,13 Furthermore, some meta-analyses performed on the subject showed no association,22,23 and others indicated that the presence of A allele was a risk factor for PD in European populations, but with marginal confidence interval.24
Our data indicated that the A allele has a significant protective effect against PD. Although our results are contradictory with some of the previous research, other studies corroborate this finding. One study in East Asians observed that the A allele was significantly less frequent in PD patients.14 Other research showed the AG genotype as a protective factor in Asians, but this association only reached significance among individuals with previous exposure to pesticides.25 Furthermore, studies have shown that carriers of A allele exhibit a significantly later onset of PD.26,27 Moreover, in two different studies in European and Asiatic populations, the G allele was considered a risk factor for Alzheimer´s disease, which shares pathological characteristics with PD.28,29
A possible mechanism for this protective role could be the fact that BDNF is synthesized as the precursor form proBDNF, which is cleaved to produce the mature form. Besides being an intermediary compound, proBDNF is a biologically active form that exerts the opposite function of the mature form. Specifically, proBDNF induces long-term depression and apoptosis through binding to p75 receptor, while the mature form promotes neuronal survival and long-term potentiation by binding to the TrkB receptor.30 Chen and colleagues showed that activity-dependent secretion is the main form of BDNF release and that most of it is secreted as proBDNF, which is cleaved into mature BDNF.5 Additionally, it was demonstrated that the amount of BDNF in this secretion pathway is diminished by the presence of at least one copy of the A allele, although total amount of BDNF in the brain is not altered.2 Therefore, the reduction of proBDNF caused by the polymorphism may confer protection, particularly in a brain that is under the neurodegenerative process of PD.31
Truncated BDNF is another form of BDNF, which is obtained through proteolytic cleavage in a different site of the proBNDF. The function of this product is unknown.30 Carlino et al. hypothesized that this isoform is an inactive form of proBDNF or that truncated BDNF inactivates proBDNF through the formation of inactive heterodimers.30 Thus, another possibility is that extracellular processing of proBDNF in truncated or mature BDNF could be influenced by the presence of the G allele, and an alteration in the proportion of these isoforms could lead to higher susceptibility to PD.30,31
Most of genetic association studies on PD and the polymorphism BDNF Val66Met have been conducted in European and Asiatic populations, with only one study being conducted in South America.32 It is known that the frequency of A allele varies greatly in the world population, being nearly absent in Sub-Saharan Africa and in some American indigenous populations and reaching 72% in She people, an ethnic group in China.33 On the one hand, even though Asians show a higher percentage of the A allele, the effects associated with this polymorphism are more consistent among Caucasians.34 This suggests that some populations could have developed a compensatory mechanism to the effects of the polymorphism. Our study found a protective association between the presence of BDNF Val66Met (allele A) polymorphism and PD. It is possible that such association is visible in the Brazilian population because it is one of the most heterogeneous populations in the world. This mixture is the result of five centuries of crosses between different ethnic groups, from European colonizers (mainly Portugal) to African slaves (west coast of Africa) and native indigenous tribes.35 Such heterogeneity creates a particular genetic background in which the polymorphism could generate a linkage disequilibrium with another polymorphism in the same gene or with other genetic variation in a gene near BDNF, causing the association effect found in this population.29 On the other hand, regarding race-related genetic factors, the ethnical heterogeneity of the Brazilian population is a limitation because of the lack of a coherent biological category. Nevertheless, apart from Brazil, there are a significant number of countries with a multiracial profile, which reinforces the need of genetic association studies in heterogenous populations.
Importantly, the present results also showed that the G/G genotype predisposes PD patients to more severe anxiety and depressive symptoms. According to the neurotrophic theory, impaired neurogenesis and neuronal plasticity are etiological factors for the development of psychiatric disorders induced by stress, such as depression and anxiety.36,37 It has been shown that decreased BDNF secretion is related to major depressive disorder in humans and that antidepressant treatments normalize the levels of this neurotrophin.37 Nonetheless, this theory has been reevaluated with the recent findings in animal and human studies. Govindarajan and colleagues observed that mice that overexpressed BDNF showed an increase in spine density in the basolateral amygdala, which is indicative of chronic stress and strongly correlated with anxiety. In contrast, BDNF overexpression also protected mice from stress-induced atrophy in hippocampal CA3 area, as well as decreased immobility in the forced swim test, suggesting that BDNF could exert different effects on different areas of the brain.38 Recently, a paper demonstrated the relation between increased BDNF in the nucleus accumbens and reduced social interaction in mice submitted to chronic social defeat stress.39 Moreover, Krishnan et al. showed that mice with G/G genotype for BDNF Val66Met polymorphism demonstrated depressive-like behavior, while A/A genotype mice exhibited resiliency, performing similar to control animals when submitted to the social defeat protocol.40 Therefore, these findings support our results, in which PD patients with G/G genotype had higher risk for more severe depression and anxiety symptoms in association with other variables.
Our study has some limitations, such as the small number of participants in comparison with other polymorphism studies. However, the number of patients in this study is consistent with official diagnostic cases in the reference hospital for movement disorders where the data collection was performed. Furthermore, power analyses showed 81% and 99% power of the detection to the significant binary logistic regressions. Thus, the associations between allele A and a protective action against PD, as well as between G/G genotype and increased risk of anxiety and depression in PD patients, are reliable.
Another limitation, as mentioned above, is the fact that this polymorphism varies greatly between ethnic populations, so it would be interesting to have included this information in the analysis. Nonetheless, Brazil has a high rate of miscegenation, so that the phenotypic characterization of ethnicity would be subjective.
Finally, the PD group of the present study included a higher proportion of those with early onset and also with positive family history than values expected in a PD sample. These two observations could indicate that most of the patients are genetically predisposed to the disease. Nevertheless, the analyses did not show genetic or clinical differences among PD subgroups (early onset, family history), at least regarding the BDNF polymorphism studied here. In this respect, an interesting question would be if this pattern of earlier age onset and positive family history of PD is common in the Brazilian population. To our knowledge, however, there are no surveys that specify proportion of such PD subgroups in Brazil. This reinforces the need for more epidemiological and genetic association studies on PD in Brazil.
Conclusions
We report an association between G/G genotype of BDNF Val66Met polymorphism and PD as well as severity of depression and anxiety. This finding provides evidence that alterations caused by this polymorphism may confer susceptibility for the development of PD. Importantly, studies that investigate associations between polymorphisms and specific symptoms of PD (especially nonmotor ones) are rare, and the present study indicates a relevant genetic factor contributing to the appearance of PD comorbidities depression and anxiety.
1 : Parkinson’s disease. First of two parts. N Engl J Med 1998; 339:1044–1053Crossref, Medline, Google Scholar
2 : Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006; 314:140–143Crossref, Medline, Google Scholar
3 : Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci 2005; 25:6251–6259Crossref, Medline, Google Scholar
4 : Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem 1992; 59:99–106Crossref, Medline, Google Scholar
5 : Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 2005; 25:6156–6166Crossref, Medline, Google Scholar
6 : Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 2003; 23:6690–6694Crossref, Medline, Google Scholar
7 : The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry 2015; 20:916–930Crossref, Medline, Google Scholar
8 : BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson’s disease. Eur J Neurol 2009; 16:1240–1245Crossref, Medline, Google Scholar
9 : The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson’s disease. J Neurol 2005; 252:833–838Crossref, Medline, Google Scholar
10 : BDNF Val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2009; 80:141–144Crossref, Medline, Google Scholar
11 : Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. Ann Neurol 2002; 51:133–136Crossref, Medline, Google Scholar
12 : Brain-derived neurotrophic factor gene polymorphisms in Japanese patients with sporadic Alzheimer’s disease, Parkinson’s disease, and multiple system atrophy. Mov Disord 2005; 20:1031–1033Crossref, Medline, Google Scholar
13 : Lack of association between the BDNF Val66Met polymorphism and Parkinson’s disease in a Swedish population. Ann Neurol 2003; 53:823Crossref, Medline, Google Scholar
14 : Association between a polymorphism of brain-derived neurotrophic factor gene and sporadic Parkinson’s disease. Ann Neurol 2003; 54:276–277Crossref, Medline, Google Scholar
15 : Val66Met BDNF polymorphism is associated with Parkinson’s disease cognitive impairment. Neurosci Lett 2016; 615:88–91Crossref, Medline, Google Scholar
16 : Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005; 180:95–99Crossref, Medline, Google Scholar
17 : Association between the BDNF Val66Met polymorphism and chronicity of depression. Psychiatry Investig 2013; 10:56–61Crossref, Medline, Google Scholar
18 : What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 1992; 42:1142–1146Crossref, Medline, Google Scholar
19 : Cellwise residual analysis in two-way contingency tables. Educ Psychol Meas 2003; 63:825–839Crossref, Google Scholar
20 : SNPStats: a web tool for the analysis of association studies. Bioinformatics 2006; 22:1928–1929Crossref, Medline, Google Scholar
21 : G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–191Crossref, Medline, Google Scholar
22 : The role of G196A polymorphism in the brain-derived neurotrophic factor gene in the cause of Parkinson’s disease: a meta-analysis. J Hum Genet 2005; 50:560–566Crossref, Medline, Google Scholar
23 : Meta-analysis study on the role of BDNF Val66Met polymorphism in Parkinson’s disease. Rejuvenation Res 2015; 18:40–47Crossref, Medline, Google Scholar
24 : BDNF 196 G/A and 270 C/T polymorphisms and susceptibility to Parkinson’s disease: a meta-analysis. J Mot Behav 2014; 46:59–66Crossref, Medline, Google Scholar
25 : Lrrk2 S1647T and BDNF V66M interact with environmental factors to increase risk of Parkinson’s disease. Parkinsonism Relat Disord 2011; 17:84–88Crossref, Medline, Google Scholar
26 : BDNF genetic variants are associated with onset age of familial Parkinson disease: GenePD Study. Neurology 2005; 65:1823–1825Crossref, Medline, Google Scholar
27 : BDNF G196A (Val66Met) polymorphism associated with cognitive impairment in Parkinson’s disease. Neurosci Lett 2014; 561:86–90Crossref, Medline, Google Scholar
28 : Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer’s disease. Mol Psychiatry 2002; 7:136–137Crossref, Medline, Google Scholar
29 : Brain-derived neurotrophic factor gene polymorphisms and Alzheimer’s disease. J Neural Transm (Vienna) 2005; 112:703–711Crossref, Medline, Google Scholar
30 : Low serum truncated-BDNF isoform correlates with higher cognitive impairment in schizophrenia. J Psychiatr Res 2011; 45:273–279Crossref, Medline, Google Scholar
31 : The Met66 allele of the functional Val66Met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Ann Rheum Dis 2006; 65:1330–1335Crossref, Medline, Google Scholar
32 : Exploration of genetic susceptibility factors for Parkinson’s disease in a South American sample. J Genet 2010; 89:229–232Crossref, Medline, Google Scholar
33 : Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry 2010; 15:810–815Crossref, Medline, Google Scholar
34 : Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci 2006; 6:79–85Crossref, Medline, Google Scholar
35 : The ancestry of Brazilian mtDNA lineages. Am J Hum Genet 2000; 67:444–461Crossref, Medline, Google Scholar
36 : Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 2002; 17(Suppl 3):306–310Crossref, Medline, Google Scholar
37 : The effect of depression, BDNF gene Val66met polymorphism and gender on serum BDNF levels. Brain Res Bull 2010; 81:61–65Crossref, Medline, Google Scholar
38 : Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 2006; 103:13208–13213Crossref, Medline, Google Scholar
39 : Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 2014; 17:27–29Crossref, Medline, Google Scholar
40 : Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131:391–404Crossref, Medline, Google Scholar



