Anxiety Levels Are Independently Associated With Cognitive Performance in an Australian Multiple Sclerosis Patient Cohort
Abstract
Neurological and psychological symptoms in multiple sclerosis can affect cognitive function. The objective of this study was to explore the relationship between psychological measures and cognitive performance in a patient cohort. In 322 multiple sclerosis patients, psychological symptoms were measured using the Depression Anxiety and Stress Scale, and cognitive function was evaluated using Audio Recorded Cognitive Screen. Multifactor linear regression analysis, accounting for all clinical covariates, found that anxiety was the only psychological measure to remain a significant predictor of cognitive performance (p<0.001), particularly memory function (p<0.001). Further prospective studies are required to determine whether treatment of anxiety improves cognitive impairment.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system, resulting in neurological and psychological symptoms. Cognitive impairment is a common feature of the disease across all disease stages and is experienced by up to 65% of patients.1 Psychological symptoms and cognitive impairment have both been shown to affect a patient’s overall quality of life and employment status.2 There is a complex interplay between these symptoms, such that psychological symptoms have been shown to affect cognitive functioning in MS, with depression, in particular, having been identified as a potentially confounding factor.3,4 However, to date, the impact of other mood indices, such as anxiety and stress, on cognitive function has received less attention.
Depression has been the most commonly evaluated psychological symptom in MS. Depressive symptoms have been reported in over 50% of patients with MS,5 three times higher than in the general population, and they occur more frequently in those with progressive disease.5 Depressed MS patients have been shown to have impaired memory, attention, and information- processing speed,3,6,7 and studies have shown that subjective impairments in cognitive function reported by depressed patients with MS improve after antidepressant treatment, independent of changes in objective cognitive performance.4,8
In addition to depression, anxiety disorders are common in MS. The lifetime prevalence of anxiety disorders in MS has been estimated at 36%, which is substantially higher than the 5% seen in the general population.9 Most constitute general anxiety disorders, whereas other common diagnoses comprise obsessive-compulsive disorder and panic disorders.10 Other investigations of the point prevalence of anxiety disorders in MS patients have identified the occurrence of other related disorders including panic disorder, social phobia, and posttraumatic stress disorder.11
Anxiety is seen more frequently in female patients with a comorbid diagnosis of depression.10 Despite the high frequency of this symptom in MS patients, there is limited information regarding the impact of this symptom on cognitive function. A recent study12 has suggested that MS patients with symptoms of state anxiety had greater impairment of attention and information-processing speed. Indeed, the effects of anxiety on cognition may well be more extensive, as studies of the effect of trait anxiety on non-MS individuals have shown effects on neural efficiency of working memory,13 and induction of an anxious state can impede verbal and spatial memory.14
There is also a growing body of evidence to support an effect of stress on MS disease course.15–17 Negative stressful events have been associated with an increase in MS lesion load,15 while behavioral stress management strategies have been shown to reduce lesion formation.16 A direct impact of stress on cognitive function in MS has not been widely examined, although altered cortisol release may be involved. Stress is known to enhance activity of the hippocampal pituitary adrenal axis, resulting in increased glucocorticoid release,17 and dysregulation of this pathway has been demonstrated in MS patients.18 In healthy individuals, a link between cortisol and long-term memory is seen,17 and when cortisol function is impaired, as in posttraumatic stress disorders, memory function is affected.19
In the current study, we have undertaken a cross-sectional, retrospective study, in our MS outpatient clinic cohort, to examine the relationship between cognitive function and psychological symptoms. The frequency of depression, anxiety, and stress occurring in our patient group was evaluated. Using predictive statistical modeling, we explored the contribution of each mood index on cognitive performance to evaluate which symptom was having the greatest impact on cognitive function in our MS clinic cohort.
Methods
Participants
MS patients who attended the outpatient clinic at the John Hunter Hospital, Newcastle, Australia were invited to participate in this study and were recruited serially. Participants consented to being assessed clinically every 6 months with the Expanded Disability Status Scale (EDSS) and to undergo psychological and cognitive evaluations annually. The study was approved by the Hunter New England Area Health HREC.
This was a retrospective, cross-sectional, observational study of a clinical cohort comprised of 322 MS patients, enrolled through the John Hunter Hospital (Newcastle, Australia) between 2008 and 2014. The data used in the current study were derived from the initial evaluation of cognitive performance and mood status performed on each participant, recruited from the time these assessments were initiated in the clinic, as part of routine clinical practice in 2008, until the time of data analysis in 2014. Neurological evaluation of patients was performed by J.L.S., and an MS diagnosis was assigned using the McDonald criteria.20 Patients were included in the study if there was no evidence of any concomitant condition that would prevent them from undertaking cognitive testing or impair their capacity to provide informed consent. This included, but was not restricted to, impaired dexterity in their hands, impeding their ability to write, or impairment to visual or auditory functioning that may limit their ability to testing.
Assessment of Cognitive Function
Patients were assessed for cognitive performance using the Audio Recorded Cognitive Screen (ARCS), which is a valid and reliable instrument for administering neuropsychological tests of cognitive function to unsupervised individuals.21,22 The ARCS assesses performance in the domains of memory, verbal fluency, language (object naming), visuospatial function, and attention, and elements from each domain score are used to derive an overall “global” cognitive performance score. ARCS scores were adjusted for factors known to affect cognitive performance and included the subject’s age, gender, and education status. Scaling was undertaken from algorithms derived from a large nonclinical ARCS validation study.21 The ARCS scores were adjusted based on normative data, where for each individual test, domain, or global score, the expected (i.e., normal) score was 100 with a standard deviation of 15. Cognitive impairment was defined as performance worse than 1.5 standard deviations below the average for the global ARCS score.
Assessment of Mental Health Status
The mental health status of the patients was assessed using the short version of the Depression Anxiety Stress Scales (DASS-21).23 Higher scores were indicative of higher levels of depression, stress, and anxiety. All scores derived from the 21-point scale were multiplied by 2 to enable comparison to the full 42-point DASS and determine clinical cutoffs for symptom severity.24 Scores indicative of high or severe depression or anxiety triggered a referral for subsequent psychiatric assessment and on-going clinical management of symptoms. The DASS comprise three self-report scales designed to measure the negative emotional states of depression, anxiety, and stress. It is not intended for use as a diagnostic tool or to replace a comprehensive clinical interview. In the DASS-21, each of the three scales contains seven items. In completing the questionnaire, the individual indicates the presence of each symptom during the previous week and applies a score ranging from 0 (did not apply at all over the last week) to 3 (applied to me most of the time over the last week). The Depression scale assesses dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest/involvement, anhedonia, and inertia. The Anxiety scale assesses autonomic arousal, skeletal muscle effects, situational anxiety, and subjective experience and anxious effect. The Stress scale is sensitive to levels of chronic nonspecific arousal and assesses difficulty relaxing, nervous arousal, and being easily upset/agitated, irritable/overreactive, and impatient. Both the full DASS24 and the abbreviated DASS-21 versions23 have been demonstrated to show adequate convergent and discriminant validity in nonclinical samples for measuring states of depression, anxiety, and stress. Furthermore, in clinical samples,25 DASS was shown to distinguish various mood and anxiety disorders in the predicted direction and was highly correlated to other questionnaires and clinical rating measures of the three emotional states.
Statistical Analyses
Initial comparison of descriptive statistics for all clinical variables was performed using either analysis of variance or chi-squared tests, depending on whether the variables were quantitative or categorical. To allow comparison among studies, the ARCS scores were then standardized (to Z scores) using the mean and SD of the normal population reported by Schofield et al.21 Bivariate linear correlation analysis of standardized ARCS scores was performed using Pearson’s (r) coefficient tests. Because moderate outliers were observed for some of the cognitive variables, we performed a secondary check of the Pearson’s correlation analyses by using Spearman’s (rho) tests, which are robust to outlier effects. These tests yielded results showing consistent strength and significance for all bivariate tests, thus adding confidence that the Pearson’s (r) results were not affected by outliers. We then performed linear regression modeling using standardized ARCS scores as outcome variables. For each regression model, we entered each of the mental health measures as the main effect factors so as to approximate the relative contribution of each mental health factor on cognitive function while accounting for the others. Tests for normality of outcome variables were performed using Q-Q plots and Kolmogorov-Smirnov goodness-of-fit tests. These tests showed some modest deviations from normality for language and visual domains, but these were not deemed substantial enough to warrant transformation. We also included a number of potentially important covariates in each of the regression models, i.e., sex, age at initial assessment, age at diagnosis, disease duration, EDSS, treatment type, MS subtype, annual relapse rate, other mental health medications, and education level. To account for multicolinearity, and so as not to overburden each regression model with too many uninformative parameters, we chose to include all main effect factors and covariates in a step-wise fashion using an algorithm that only retained covariates if they contributed to the overall regression model (p<0.05). In an effort to discern the relative association of the three mood indices from each other, and in relation to the other clinical covariates, we partitioned our regression models to include forced entry of each mood index separately, with all other clinical covariates entered in a step-wise fashion, and forced entry of all three mood indices combined, with all other clinical covariates entered in a step-wise fashion. Because we tested six different hypotheses (i.e., six cognitive function outcomes), we used the Bonferroni correction method to adjust the significance level to 0.008 (i.e., 0.05/6). In addition, we retained results at the “suggestive” level of 0.05 to provide an indication of association trends.
Results
The descriptive characteristics for the MS subtypes are shown in Table 1. Seventy-nine percent of our cohort were classified as relapsing remitting (RRMS), 14% were secondary progressive (SPMS), and 7% primary progressive (PPMS) MS. Patients receiving MS-specific immunomodulatory treatments were predominantly RRMS patients and were receiving interferon beta (N=89), glatiramer acetate (N=42), natalizumab (N=25), fingolimod (N=9), dimethyl fumarate (N=4), or no MS immunomodulatory treatment (N=153) at the time of undertaking the study assessments. SPMS patients were older, had a longer duration of disease, and had a higher EDSS level than their RRMS counterparts at the time of the assessments. Using the severity grading criteria for each mood index on the DASS,24 the severity of psychological symptoms was evaluated. In our MS cohort, 12% of patients reported severe or extremely severe anxiety, with 14% scoring moderate levels of anxiety (Figure 1). Thirty-one percent were undergoing treatment with a serotonin reuptake inhibitor at the time of undertaking cognitive testing. Based on our definition of cognitive impairment (see above), 34% of our MS cohort were cognitively impaired.
| Variable | Relapsing Remitting MS (N=258) | Secondary Progressive MS (N=43) | Primary Progressive MS (N=21) | p |
|---|---|---|---|---|
| Demographic characteristic | ||||
| Age at initial assessment (years) | 43±12 | 57±10 | 46±13 | <0.001 |
| Sex (female) | 213 (83%) | 35 (81%) | 13 (62%) | 0.07 |
| More than 12 years of educationb | 29% | 14% | 19% | 0.09 |
| Disease characteristic | ||||
| Age at diagnosis (years) | 36±11 | 41±11 | 55±8 | <0.001 |
| Disease duration | 7±7 | 15±10 | 7±6 | <0.001 |
| Expanded Disability Status Scale score | 3±2 | 6±1 | 6±1 | <0.001 |
| Annual relapse rate | 1±1 | NA | NA | NA |
| MS treatment (yes) | 152 (59%) | 15 (35%) | 2 (10%) | 0.000 |
| Other treatmentc (yes) | 69 (27%) | 19 (44%) | 10 (48%) | 0.016 |
| Cognitive performanced | ||||
| Memory score | 84±22 | 80±26 | 92±18 | 0.15 |
| Fluency score | 84±17 | 81±16 | 93±17 | 0.02 |
| Visual score | 97±15 | 92±24 | 105±14 | 0.02 |
| Language score | 88±20 | 90±29 | 98±14 | 0.08 |
| Attention score | 91±15 | 85±18 | 102±16 | <0.001 |
| Overall Audio Recorded Cognitive Screen score | 82±19 | 78±24 | 97±17 | 0.01 |
| Mental health | ||||
| Depression score | 8±9 | 9±8 | 10±11 | 0.64 |
| Anxiety score | 6±7 | 7±8 | 6±9 | 0.63 |
| Stress score | 12±10 | 10±8 | 9±11 | 0.19 |
| Overall Depression Anxiety Stress Scales score | 27±23 | 27±21 | 24±27 | 0.91 |
TABLE 1. Characteristics of the Multiple Sclerosis (MS) Cohorta
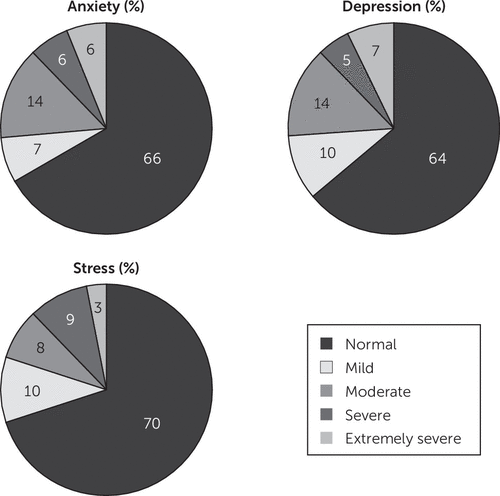
FIGURE 1. Distribution of Mood Indices in the Multiple Sclerosis (MS) Cohort Showing Proportions of Patients at Each Level of Severitya
a Gradings of symptom severity were based on the 42-point Depression Anxiety Stress Scales clinical cutoffs for symptom severity.27
There were several differences for the cognitive performance variables between MS subtypes (p<0.05). In general, at the group level, SPMS patients scored lower and PPMS patients higher for the overall ARCS score than those with RRMS. There were no statistically significant differences between MS subtypes for the mental health variables (p>0.1). While there was no significant difference between male and female patients for these variables, males tended to have lower average memory scores compared with females (p=0.07).
Cognitive performance variables (ARCS scores) were converted to Z scores for bivariate correlation and regression analyses (see the Methods section). Bivariate analyses showed widespread negative correlations between clinical variables and the cognitive performance variables (p<0.05) (Table 2). The strongest negative correlation was observed between memory score and EDSS (r=−0.35, p=0.001). There was a general tendency for a lower cognitive performance, across all domains, to correlate with higher levels of stress, anxiety, and depression. The strongest individual mental health correlate of overall cognitive ability was anxiety (r=−0.27, p=0.004). Among the cognitive domains, anxiety also showed the strongest correlation with memory (r=−0.27, p=0.0005). The level of disability accumulation, as measured by EDSS, showed a weak correlation with both anxiety and depression indices (data not shown).
| Variable | Memory | Fluency | Visual | Language | Attention | Overall Audio Recorded Cognitive Screen Scores |
|---|---|---|---|---|---|---|
| Disease | ||||||
| Age at assessment (years) | –0.34** | –0.27** | –0.17** | 0.08 | –0.35** | –0.27** |
| Age at diagnosis (years) | –0.21** | –0.15* | –0.06 | 0.15** | –0.20** | –0.11 |
| Disease duration | –0.24** | –0.18** | –0.20** | –0.10 | –0.26** | –0.27** |
| Expanded Disability Status Scale score | –0.35** | –0.33** | –0.20** | –0.08 | –0.33** | –0.35** |
| Annual relapse rate | 0.13* | 0.18** | 0.10 | –0.11 | 0.18** | 0.12* |
| Mental health | ||||||
| Stress | –0.12* | –0.07 | –0.10 | –0.08 | –0.13* | –0.15* |
| Anxiety | –0.28** | –0.21** | –0.17** | –0.11 | –0.22** | –0.27** |
| Depression | –0.21** | –0.13* | –0.12* | –0.03 | –0.19** | –0.18** |
| Overall Depression Anxiety Stress Scales score | –0.22** | –0.15** | –0.14* | –0.08 | –0.20** | –0.22** |
TABLE 2. Bivariate Correlation of Cognitive Performance Variablesa
To assess the relative association of each of the mental health variables (depression, anxiety and stress) with cognitive ability in this MS patient cohort, we performed linear regression analyses factoring in these three main factors as well as relevant covariates. When analyzed separately, all three mood indices were significantly associated with overall cognitive performance, after accounting for significant clinical covariates (p<0.008). Interestingly, when we modeled all three mood indices together, anxiety was the only mental health variable that was significantly associated with cognitive function (Table 3). After conditioning on stress and depression, increased anxiety scores were significantly associated with reduced cognitive performance (β=−0.22, p<0.008). These results indicate that the association of stress and depression with cognitive outcome is no longer statistically significant in this model after accounting for the association with anxiety. Analyses of cognitive subdomains showed that the association of anxiety appeared to exist across the different cognitive domains but was strongest for memory (β=−0.22, p<0.008). Of all regression models, the memory model yielded the highest explanatory value showing that, in combination with sex, age, and EDSS, anxiety explains 24% of the variance in memory domain (adjusted R2=0.24, p<0.008).
| Variables | Memory | Fluency | Visual | Language | Attention | Overall Audio Recorded Cognitive Screens | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | |
| Main factors | ||||||||||||
| Stress | — | — | — | — | — | — | — | — | — | — | — | — |
| Anxiety | –0.22 | <0.008 | –0.16 | <0.008 | –0.015 | 0.01 | –0.11 | 0.047 | –0.15 | <0.008 | –0.22 | <0.008 |
| Depression | — | — | — | — | — | — | — | — | — | — | — | — |
| Covariates | ||||||||||||
| Sex | –0.25 | <0.008 | –0.19 | <0.008 | — | — | — | — | — | — | –0.14 | 0.01 |
| Age (years) | –0.17 | <0.008 | — | — | — | — | — | — | –0.24 | <0.008 | — | — |
| Age at diagnosis (years) | — | — | — | — | — | — | — | — | — | — | — | — |
| Disease duration | — | — | — | — | –0.15 | 0.013 | –0.14 | 0.02 | — | — | –0.17 | <0.008 |
| Treatment | — | — | — | — | — | — | 0.15 | 0.01 | — | — | — | — |
| Multiple sclerosis subtype | — | — | — | — | — | — | — | — | — | — | — | — |
| Expanded Disability Status Scale score | –0.21 | <0.008 | –0.29 | <0.008 | –0.15 | 0.017 | — | — | –0.16 | 0.01 | –0.24 | <0.008 |
| Other medications | — | — | — | — | –0.14 | 0.016 | — | — | — | — | — | — |
| Annual relapse rate | — | — | — | — | — | — | –0.13 | 0.03 | — | — | — | — |
| Adjusted R2 | 0.24 | <0.008 | 0.15 | <0.008 | 0.08 | <0.008 | 0.05 | <0.008 | 0.15 | <0.008 | 0.19 | <0.008 |
TABLE 3. Regression Models for Cognitive Performance Outcomesa
Discussion
Recently, cognitive impairment has received increased attention by MS experts, and there is a trend to establish monitoring of cognitive deficits as part of routine clinical practice in MS clinics. It is important to consider the impact of other potentially confounding factors on cognitive function at the time of assessment. Of the psychological parameters that have been evaluated, there have been extensive reports detailing an association between depression and cognitive performance in MS patients.3,4 Indeed, in our study we also see a relationship between the level of depression and cognitive function, with depression scores being inversely associated with performance on memory, fluency, and attention functional domains. In contrast, the impact of anxiety disorders in MS has received less investigation to date, despite their occurrence in about one third of patients.9 In our clinic cohort of 322 MS patients, 26% reported anxiety of at least moderate severity. In a large study of patients with MS,10 symptoms of anxiety were closely associated with suicide attempts. The finding that suicide is the cause of 15% of all deaths in MS'26 highlights the need for routine monitoring of anxiety symptoms within this patient population.
We found that anxiety had a significant association with cognitive performance after accounting for the association of depression and stress, and this relationship among mood indices was consistent, regardless of the varying associations of the other clinical covariates. This suggests that in this cohort the association with anxiety may be of the highest relative importance for cognitive function among the three mood indices measured. To our knowledge, this is the largest study objectively assessing anxiety and its association with cognition in MS. This study is also unique in its assessment of the contribution of depression, anxiety, and stress on overall cognitive function, as well as five cognitive domains to evaluate which mood index has the most dramatic impact on cognitive performance in MS patients. Our findings support those shown by Goretti et al.,12 in which the impact of psychological symptoms on cognitive impairment in 190 patients was evaluated. They showed state anxiety to be stronger than depression (or fatigue) as a predictor of cognitive function, when defining anxiety symptoms using the State-Trait Anxiety Inventory and depression by the Beck Depression Inventory. Delineation of anxiety disorder subtypes was not applied in the current study; rather the identification of psychological symptoms was by application of the DASS-21 self-report questionnaire. This approach enabled symptoms of anxiety to be identified at the time of undertaking the cognitive testing and provided findings comparable to those observed in a study by Goretti et al.,12 in which anxiety was seen to be a stronger predictor of cognitive performance than depression. Our clinic cohort was comprised predominantly of RRMS patients (79%) with an average disease duration of 7 years but also included patients with a progressive disease phenotype (21%). This differed slightly from the Goretti et al. 12 study, which was restricted to RRMS patients with a longer average disease duration. However, the association of anxiety with cognitive performance is not restricted to RRMS cases, as in early MS and in clinical isolated syndrome, increased anxiety scores have also been associated with cognitive impairment.27 In our study, we did not see any association between duration of disease with the magnitude of psychological symptoms, hence the severity of anxiety was seen to a similar extent in both the early stages and long-term disease.
The major association of anxiety with cognitive performance in our clinic cohort was with respect to memory and fluency. The cognitive testing battery we applied in our study was the ARCS, which—while it differs from those used by other investigators—is sensitive and obtains results consistent with those derived from a comprehensive assessment by a neuropsychologist and also correlates closely with the more commonly used tool SDMT.22 Conceivably, utilization of different cognitive tests may account for differences in functional domains associated with anxiety seen in other studies. Goretti et al. 12 applied Rao’s Brief Repeatable Battery and found that state anxiety was associated with poorer performance on complex attention tasks and information-processing speed. Julian and Arnett28 also demonstrated a stronger association of state anxiety than depression on cognitive function, particularly for tasks measuring “executive functioning” (the Shipley Vocabulary Test and Visual Elevator Subtest).
The mechanisms that underpin the association of increased anxiety with poorer performance on cognitive tests in patients with MS are not clear. A first, obvious possibility, is that with increasing lesion load, structures relevant to anxiety and cognition are both increasingly likely to be damaged.29 Imaging studies point to the involvement of, and altered connectivity with, subcortical gray matter structures such as the amygdala, even relatively early during the course of MS.30,31 A second possibility is that a patient’s perception of loss of cognitive or general functioning might make them anxious. In our study, the level of disability accumulation, as measured by EDSS, was correlated with the level of anxiety and depression, consistent with findings from a large online U.K. registry study.32 Indeed, findings from the U.K. study suggest that physical disability is a major predictor of anxiety and depression in MS, although more subjective evaluations of disability were applied compared with the EDSS evaluations conducted in the current study. Clearly, the first mechanism mentioned could also account for these associations. Third, anxiety might have a more direct consequence for cognitive functioning, independent of any considerations of lesion load. In relation to this third mechanism, studies conducted in threat of shock studies or in non-MS anxiety disorders,33 anxiety has been shown to affect attention, working memory, and executive function. The role of anxiety on working memory has also been supported by functional MRI (fMRI) in individuals with trait anxiety.13 High signal in the right dorsolateral prefrontal cortex and left inferior frontal sulcus on fMRI correlated with high levels of anxiety and was considered to be related to impairment in processing efficiency in these individuals. Fourth, anxiety has also been associated with other behavioral issues that themselves may affect disease outcome and cognitive performance in MS. Alcohol dependence and smoking have been associated with anxiety and depression in MS.34 Such behaviors may in turn be potentially impactful on MS disease progression35 and subsequently cognitive function. Finally, depression or anxiety disorders in MS have been linked to specific personality traits in individuals with MS to include those with more neuroticism, introversion, less agreeableness, and less conscientiousness.36 These traits have been linked directly to impairment of cognitive function, with MS patients displaying low conscientiousness and high neuroticism having more brain atrophy, evidenced by reduced gray matter volume, and impaired information-processing speed.37
In addition to showing the association of depression and anxiety with cognitive performance, we also saw that stress correlated with cognitive function. However, the correlations between stress scores and cognitive domains were more modest than those for depression and anxiety.
Our study has some important limitations. Our measures of anxiety and depression were obtained by questionnaire and were not supplemented by any formal clinical diagnoses. Nevertheless, accepted cutoffs on the screening measures were used to define meaningful categories of severity. The cross-sectional nature of the study prevents us from making any causal inferences. Indeed, as we have indicated, the potential mechanisms of association between anxiety and cognition are numerous and complex. Although identification and treatment of anxiety (and depression) are clinically appropriate, we lack definitive evidence to suggest that such treatment would necessarily also benefit cognition. Our evaluation of the association of stress with cognitive function was also limited and did not enable an identification of the frequency or nature of stressful life events that may have occurred. These have been suggested by others to be key factors in understanding the impact of stress on MS symptoms and disease progression.16
In conclusion, depression, anxiety, and stress are highly prevalent in MS patients. Anxiety is significantly associated with cognitive performance, independent of depression in MS, and future studies are needed to determine whether treatment of anxiety also benefits cognition in these patients.
1 : Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 2006; 245:41–46Crossref, Medline, Google Scholar
2 : Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health 2012; 15:1029–1035Crossref, Medline, Google Scholar
3 : Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol 2008; 23:189–199Crossref, Medline, Google Scholar
4 : Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology 2010; 24:573–580Crossref, Medline, Google Scholar
5 : A large-scale study of anxiety and depression in people with multiple sclerosis: a survey via the web portal of the UK MS Register. PLoS One 2012; 7:e41910Crossref, Medline, Google Scholar
6 : Presence and significant determinants of cognitive impairment in a large sample of patients with multiple sclerosis. PLoS One 2013; 8:e69820Crossref, Medline, Google Scholar
7 : Apathy/depression, but not subjective fatigue, is related with cognitive dysfunction in patients with multiple sclerosis. BMC Neurol 2014; 14:3Crossref, Medline, Google Scholar
8 : The relationship between self-reported executive performance and psychological characteristics in multiple sclerosis. Eur J Neurol 2012; 19:562–569Crossref, Medline, Google Scholar
9 : Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disorder 2009; 2:13–29Crossref, Medline, Google Scholar
10 : Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler 2007; 13:67–72Crossref, Medline, Google Scholar
11 : Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler 2009; 15:1518–1524Crossref, Medline, Google Scholar
12 : Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci 2014; 35:559–563Crossref, Medline, Google Scholar
13 : Trait anxiety and the neural efficiency of manipulation in working memory. Cogn Affect Behav Neurosci 2012; 12:571–588Crossref, Medline, Google Scholar
14 : The complex interaction between anxiety and cognition: insight from spatial and verbal working memory. Front Hum Neurosci 2013; 7:93Crossref, Medline, Google Scholar
15 : Do positive or negative stressful events predict the development of new brain lesions in people with multiple sclerosis? Psychol Med 2014; 44:349–359Crossref, Medline, Google Scholar
16 : A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology 2012; 79:412–419Crossref, Medline, Google Scholar
17 : Stress and memory in humans: twelve years of progress? Brain Res 2009; 1293:142–154Crossref, Medline, Google Scholar
18 : Stress and hypothalamic-pituitary-adrenal axis function in experimental autoimmune encephalomyelitis and multiple sclerosis—a review. Psychoneuroendocrinology 2007; 32:604–618Crossref, Medline, Google Scholar
19 : Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder—2014 Curt Richter Award Winner. Psychoneuroendocrinology 2015; 51:282–295Crossref, Medline, Google Scholar
20 : Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69:292–302Crossref, Medline, Google Scholar
21 : The Audio Recorded Cognitive Screen (ARCS): a flexible hybrid cognitive test instrument. J Neurol Neurosurg Psychiatry 2010; 81:602–607Crossref, Medline, Google Scholar
22 : The Audio Recorded Cognitive Screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics. Mult Scler 2010; 16:1126–1133Crossref, Medline, Google Scholar
23 : The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 2005; 44:227–239Crossref, Medline, Google Scholar
24 Lovibond SH, Lovibond PF: Manual for the Depression Anxiety Stress Scales. 2. Sydney, Australia, Psychology Foundation, 1995Google Scholar
25 : Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behav Res Ther 1997; 35:79–89Crossref, Medline, Google Scholar
26 : Cause of death in patients attending multiple sclerosis clinics. Neurology 1991; 41:1193–1196Crossref, Medline, Google Scholar
27 : Cognition, mood and fatigue in patients in the early stage of multiple sclerosis. Swiss Med Wkly 2007; 137:496–501Medline, Google Scholar
28 : Relationships among anxiety, depression, and executive functioning in multiple sclerosis. Clin Neuropsychol 2009; 23:794–804Crossref, Medline, Google Scholar
29 : Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol 2009; 68:489–502Crossref, Medline, Google Scholar
30 : Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry 2010; 81:690–695Crossref, Medline, Google Scholar
31 : Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain 2009; 132:3380–3391Crossref, Medline, Google Scholar
32 : Physical disability, anxiety and depression in people with MS: an internet-based survey via the UK MS Register. PLoS One 2014; 9:e104604Crossref, Medline, Google Scholar
33 : The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci 2013; 7:203Crossref, Medline, Google Scholar
34 : Adverse health behaviours are associated with depression and anxiety in multiple sclerosis: a prospective multisite study. Mult Scler J 2016; 16:685–693Crossref, Google Scholar
35 : Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disorder 2012; 5:13–22Crossref, Medline, Google Scholar
36 : Personality traits in multiple sclerosis: association with mood and anxiety disorders. J Psychosom Res 2011; 70:479–485Crossref, Medline, Google Scholar
37 : Influence of personality on the relationship between gray matter volume and neuropsychiatric symptoms in multiple sclerosis. Psychosom Med 2013; 75:253–261Crossref, Medline, Google Scholar



