Functional and Structural Connectivity of the Cerebellar Nuclei With the Striatum and Cerebral Cortex in First-Episode Psychosis
Abstract
Objective:
Evidence suggests that the cortico-striatal-thalamo-cortical circuitry plays an important role in schizophrenia pathophysiology. Cerebellar contribution from deep cerebellar nuclei to the circuitry has not yet been examined. The authors investigated resting-state functional connectivity (RSFC) of cerebellar output nuclei with striatal-thalamic-cortical regions in relation to white-matter integrity and regional gray-matter volumes in first-episode psychosis (FEP).
Methods: Forty FEP patients and 40 age- and gender-matched healthy control subjects (HCs) participated. RSFC between cerebellar nuclei and striatal-thalamic-cortical regions was examined. Diffusion tensor imaging and volumetric scans were examined for possible structural constraints on RSFC. The authors also examined relationships between neuroimaging variables and cognitive and clinical measures.
Results: FEP patients, compared with HCs, exhibited decreased RSFC between the left fastigial nucleus and right putamen, which was associated with poor letter fluency performance and lower global assessment of functioning scores. By contrast, patients showed widespread increased accumbens network connectivity in the left nucleus. The authors further observed both hypo- and hyper-RSFC between the cerebellar nuclei and fronto-parietal areas in patients, independent of striatal activity. Finally, the authors found impaired integrity of the left superior cerebellar peduncle and decreased bilateral putamen volume in patients, whereas structural-functional relationships found in HCs were absent in patients.
Conclusions: This study provides evidence of disordered RSFC of cerebellar output nuclei to the striatum and neocortex at the early stage of schizophrenia. Furthermore, dysfunctional cerebellar influences on fronto-parietal areas that are independent of striatal dysfunction in patients with FEP were observed. The results suggest that cortico-striatal abnormalities in patients with FEP are produced by abnormal cerebellar influences.
The striatum as part of the cortico-striato-thalamo-cortical circuitry may play a central role in schizophrenia pathophysiology, because the striatum is the primary target of all antipsychotics.1 The dorsal striatum is associated with cognitive and motor functions in conjunction with dorsolateral prefrontal and sensorimotor areas, while the ventral striatum is related to emotional functions via its connections with limbic and orbitofrontal regions.2 Excessive yet imbalanced dopaminergic activities in the striatum along the dorsal and ventral axis appear to be critical in understanding schizophrenia pathophysiology.3 Nonetheless, the exact nature of striatal dysfunction in schizophrenia is still illusive, and it may be necessary but not sufficient to explain the neurobiological heterogeneity of schizophrenia.
Cerebellar efferents have their origin in the deep cerebellar nuclei, and they reach multiple subdivisions of the thalamus via the superior cerebellar peduncle (SCP) to modulate the cortico-striato-thalamo-cortical circuitry.4 The dentate nucleus (DN), which is under the lateral hemisphere, is divided into dorsal and ventral portions (DNd and DNv), as DNd mainly projects to cortical motor areas via the motor thalamus whereas DNv is predominantly connected to prefrontal cognitive areas via the mediodorsal thalamus. Likewise, a more medially located nucleus under the paravermal zone, the interpositus nucleus (IN) has both anticipatory motor and cognitive functions.5 Finally, the fastigial nucleus (FN) is located under the anterior vermis and has connections with ventral tegmental dopamine neurons targeting ventral striatal neurons to contribute to emotional functions.6
To date, evidence on the contribution of cerebellar efferents to the striatum, thalamus and cerebral cortex in schizophrenia is lacking. Although there have been some studies showing generally decreased resting-state functional connectivity (RSFC) between the cerebral cortex and cerebellar cortical areas in schizophrenia including antipsychotic-naïve cases.7,8 It is important to investigate cerebellar efferents, because Purkinje cells, which provide the output of the cerebellar cortex to the deep cerebellar nuclei, have been reported to be impaired in terms of reduced cell size and density in schizophrenia.9 Hence, it is likely that cerebellar input and output are dissociated. Resting-state fMRI provides an opportunity for such an investigation, as an in vivo examination of intrinsic brain organization.10
Here, we examined RSFC between cerebellar output nuclei and striatal regions using resting-state fMRI in patients with first-episode psychosis (FEP), to minimize the confounding effects of illness chronicity and long-term antipsychotic medication. In particular, we wished to determine the unique influences of each region to the whole brain that are not mediated by other regions, using a seed-to-voxel semipartial correlation analysis. We also examined the integrity of cerebellar white-matter output tracts (the SCP) using diffusion tensor imaging (DTI) and cerebellar and striatal gray-matter volumes for possible structural-functional constraints, as we sought a convergence of evidence. The functional significance of these measures would be examined using a cerebellar-sensitive cognitive measure of verbal fluency11 and clinical ratings. We hypothesized a relative hyperconnectivity between cerebellar output nuclei and striatal regions in FEP, based on evidence for excessive cerebellar activity12 and also based on hyperactivity in the striatal dopaminergic system in schizophrenia.1
Methods
Participants
Forty patients with a diagnosis of DSM-IV schizophrenia spectrum disorders with their first-episode participated (Table 1). They were from the Seoul Youth Clinic early psychosis cohort from April 2010 to June 2015. All had a history of less than a year since their first psychotic episode. Twenty-nine patients were on atypical antipsychotic medication at the time of data collection. Forty healthy participants individually matched for age (within 2 years) and gender served as a healthy control group. All participants underwent the Structured Clinical Interview for DSM-IV Axis I disorders (patient or non-patient edition). Exclusion criteria for both groups were contraindications to magnetic resonance scanning, neurological disorders (including previous head injury), and learning disabilities. After complete description of the study to the participants, written informed consent was obtained. The study was approved by the local research ethics committee.
| Variable | Patient Group (N=40) | Healthy Control Group (N=40) | Difference | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Male/female | 18/22 | 18/22 | |||
| Age (years) | 22.9 | 5.6 | 23.1 | 5.0 | |
| Education (years) | 13.3 | 2.0 | 13.8 | 1.7 | |
| Handedness (right/left) | 35/5 | 39/1 | |||
| IQ | 98.3 | 13.1 | 115.7 | 10.9 | <0.001 |
| Letter fluency | 33.1 | 10.2 | 45.1 | 9.7 | <0.001 |
| Category fluency | 31.0 | 8.7 | 43.2 | 8.6 | <0.001 |
| Antipsychotics (yes/no) | 29/11 | ||||
| Chlorpromazine equivalent dose (mg/day) | 390 | 245 | |||
| current | 45.95 | 9.7 | |||
| Global Assessment of Functioning (past year) | 70.13 | 11.9 | |||
| PANSS | |||||
| Positive | 16.5 | 4.9 | |||
| Negative | 17.6 | 5.5 | |||
| General | 35.4 | 7.2 | |||
TABLE 1. Demographic and clinical data for 40 patients with first-episode psychosis compared with healthy control subjectsa
MRI Data Acquisition
Neuroimaging data sets were acquired using a 3-T scanner (Siemens Magnetom Trio, Erlangen, Germany) at Seoul National University Hospital. We acquired 35 contiguous transverse slices at 116 time points using echo-planar imaging (TR=3,500 ms, TE=30 ms, 3.5-mm slice thickness, field of view: 240mm, in-plane matrix of 128×128, in-plane resolution 1.9×1.9mm2). Participants were asked to keep their eyes closed, but to stay awake. After scanning, participants were debriefed to confirm that they had not fallen asleep. A high resolution T1-weighted structural MRI was acquired for each participant using a 3D MPRAGE sequence (TR=1670 ms; TE=1.89 ms; 1-mm slice thickness, field of view: 250mm, in-plane matrix=256×256, in-plane resolution 0.98×0.98 mm2, 208 slices). Diffusion weighted images were acquired using echo-planar imaging (TR=1,1400 ms, TE=88 ms, matrix 128×128, field of view: 240 mm, and a voxel size of 1.9×1.9×3.5 mm3). Diffusion-sensitizing gradient echo encoding was applied in 64 directions using a diffusion-weighting factor b of 1000s /mm2. One volume was acquired with b factor of 0 seconds/mm2 (without gradient).
Resting-State Functional Connectivity Analyses
Resting-state fMRI data were analyzed using the Statistical Parametric Mapping software (SPM12, www.fil.ion.ucl.ac.uk/spm) with the Functional Connectivity toolbox (V16).13 All participants met a movement threshold of under 2.5 mm in any direction or 2.5° in any rotation. Preprocessing involved realignment, unwarping, slice-time correction, functional and anatomical images coregistration, normalization with a resampled voxel size of 2×2 × 2 mm3, and smoothing using a Gaussian kernel of full-width half-maximum 6 mm.
To minimize motion related artifacts in RSFC results, we used Friston’s 24 head motion parameters in minimizing motion related artifacts (6 head motion parameters, 6 head motion parameters for difference between one time point and its preceding time point, and the 12 corresponding squared parameters).14 The use of Friston’s 24 parameters was superior to the use of smaller sets of parameters in removing motion artifacts for resting state fMRI analysis.15 Data scrubbing (or data censoring) was not performed as it could produce a confounding effect in between-group RSFC analyses.16 This was possible, because it would make degree of freedom different among participants. Further, five principal components from each of white-matter and CSF masks were used in regressing out physiological artifacts as implemented in the CONN toolbox. The resulting time series were bandpass-filtered between 0.008 and 0.09 Hz to reduce the effect of low frequency drifts and high-frequency noise.
Region of interest (ROI) masks for striatal and thalamic regions were obtained from the Harvard-Oxford subcortical atlas (fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Masks for deep cerebellar nuclei (DNd, DNv, IN and FN) were taken from the SPM neuroanatomy toolbox. All ROIs were thresholded to contain only voxels that were inside each ROI with a probability threshold above 60%, to prevent signal contamination from neighboring regions. When extracting ROI-level signals, we used unsmoothed images to further avoid signal contamination from surrounding areas.17 These were important in using cerebellar ROIs that are close to each other.
We conducted 3 levels of analyses. First, we did ROI individual connectivity analysis using a 16×16 ROI-to-ROI connectivity matrix with false-discovery rate (FDR) corrected p values. Secondly, we used network-based statistic (NBS) to the ROI connectivity matrix to identify interconnected structures that are associated with group-differences in network intensity (sum of test statistic values across all of significant connections).18 In so doing, we first selected individual connections with a lenient threshold (p<0.1), and then we applied family-wise error (FWE) correction with a threshold of p<0.05.18 The use of these complementary methods is important in identifying damaged connections and compensatory network activity in abnormal brain function.19 Finally, we performed a seed-to-voxel semipartial correlation analysis, to investigate each ROI’s unique influence to the whole brain, controlling for other ROIs’ contributions (height threshold p<0.001 uncorrected; extent threshold p<0.05 FWE-corrected) (Figure 1). In this analysis, thalamic ROIs, whose activity would be modulated by both cerebellar and striatal activities, were omitted.
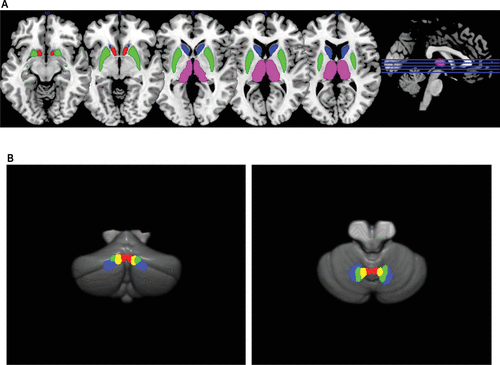
FIGURE 1. Striatal, thalamic, and cerebellar regions of interest (ROIs) used in the studya
a The following ROIs are shown in Panel A: caudate (blue), putamen (green), nucleus accumbens (red), and thalamus (pink). The following ROIs are shown in Panel B (left: posterior view, right: superior view): ventral dentate nucleus (blue), dorsal dentate nucleus (green), interpositus nucleus (yellow) and fastigial nucleus (red).
Diffusion Tensor Imaging Analyses
Diffusion weighted images were processed using FMRIB’s Diffusion toolbox (fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). Images were corrected for motion and eddy current distortions. ROIs were the left and right superior cerebellar peduncle (SCP) in a standard atlas.20 The atlas space was aligned to each participant’s T1 image using FMRIB’s Nonlinear Image Registration Tool, which was then aligned to the individual DTI image using FMRIB’s Linear Image Registration tool. As the T1 image had higher resolution, the use of each participant’s T1 image in the registration stage would result in more accurate estimation of transformation matrix. Mean values of axial diffusivity (water diffusion in the direction of the axon), radial diffusivity ([RD] water diffusion orthogonal to the axon), mean diffusivity (overall water diffusivity), and fractional anisotropy ([FA] a composite measure of the directionality of water diffusion) were extracted.
Volumetric Analyses
The structural T1-weighted images were processed using the FreeSurfer (v.5.3.0, http://freesurfer.net) to obtain striatal, thalamic and total intracranial volumes. For a detailed investigation of cerebellar subregions, we used the Spatially Unbiased Infratentorial Toolbox (SUIT version 3.1: www.diedrichsenlab.org/imaging/suit.htm). On the T1 image, the cerebellum was isolated, segmented, and normalized to the SUIT template. The inverse deformation field during the normalization step was applied to the template, to bring the template into native space and to calculate volumes of 28 cerebellar compartments within the template with respect to the isolated and gray-matter segmented image. Resulting parcellated gray-matter volumes were grouped into six regions of the cerebellar cortex (left and right of anterior, posterior superior, and posterior inferior regions) and the vermis.
Verbal Fluency and Clinical Ratings
Verbal fluency was assessed with the Controlled Oral Word Association Test (COWA).21 Patients’ functional outcome was measured using the Global Assessment of Functioning (GAF) scale of the DSM-IV. Symptoms were rated using the Positive and Negative Syndrome Scale (PANSS). We grouped PANSS items to derive the three core psychopathological dimensions of ‘psychomotor poverty’ (negative symptoms), ‘disorganization’ (thought disorder and bizarre behavior), and ‘reality distortion’ (hallucinations and delusions), as previously.22
Results
ROI Connectivity Analyses
Patients exhibited significantly decreased RSFC between left FN and right putamen ROIs, compared with HCs (t=–3.19, df=78, p=0.026, corrected) (Table 2, Figure 2). This RSFC strength was positively correlated with letter fluency performance in patients (r=0.36, p=0.021; but not in HCs: r=−0.109, p=0.539), and global assessment of functioning (GAF) scores in patients (both current and past year: r=0.38, p=0.015, and r=0.40, p=0.010).
| Seed ROI | HCs (N=40) | FEP (N=40) | HCs > FEP | Statistics | FEP>HCs | Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regions | Significance (p) | Regions | Significance | Regions | t | p | Regions | t | p | |
| DN_dorsal_L | n.s. | n.s | NAc_R | 2.32 | 0.011 | |||||
| NAc_L | 0.0050 | |||||||||
| DN_dorsal_R | Thalamus_L | 0.047 | ||||||||
| Putamen_L | 0.015 | NAc_L | 0.012 | Putamen_L | 2.23 | 0.014 | NAc_L | 1.80 | 0.038 | |
| Thalamus_L | 0.016 | Thalamus_L | 0.026 | |||||||
| DN_ventral_L | n.s | |||||||||
| Caudate_R | 0.046 | NAc_L | 0.001 | NAc_L | 2.36 | 0.011 | ||||
| Thalamus_L | 0.002 | |||||||||
| Caudate_R | 0.004 | |||||||||
| DN_ventral_R | n.s | n.s. | ||||||||
| Thalamus_L | 0.001 | Putamen_R | 0.001 | |||||||
| Caudate_L | 0.004 | Thalamus_L | 0.001 | |||||||
| Thalamus_R | 0.023 | Putamen_L | 0.005 | |||||||
| Caudate_R | 0.031 | Thalamus_R | 0.015 | |||||||
| IN_L | n.s | n.s. | ||||||||
| Caudate_R | 0.011 | NAc_L | 0.011 | |||||||
| Putamen_R | 0.020 | Thalamus_R | 0.049 | |||||||
| Thalamus_R | 0.024 | |||||||||
| IN_R | n.s | |||||||||
| Thalamus_L | 0.027 | Thalamus_L | 0.003 | NAc_R | 2.35 | 0.011 | ||||
| Thalamus_R | 0.039 | Thalamus_R | 0.006 | NAc_L | 1.84 | 0.035 | ||||
| NAc_L | 0.008 | |||||||||
| FN_L | n.s. | |||||||||
| Putamen_R | 0.000 | Thalamus_L | 0.009 | Putamen_R | 3.19 | 0.001 | ||||
| Caudate_R | 0.000 | Caudate_R | 2.39 | 0.009 | ||||||
| NAc_L | 0.007 | Putamen_L | 2.09 | 0.019 | ||||||
| Putamen_L | 0.008 | |||||||||
| Thalamus_L | 0.009 | |||||||||
| Thalamus_R | 0.014 | |||||||||
| Caudate_L | 0.023 | |||||||||
| NAc_R | 0.0473 | |||||||||
| FN_R | n.s. | |||||||||
| Thalamus_L | 0.000 | Thalamus_L | 0.008 | NAc_L | 1.79 | 0.039 | ||||
| Thalamus_R | 0.001 | Thalamus_R | 0.009 | |||||||
| Putamen_L | 0.031 | NAc_L | 0.012 | |||||||
TABLE 2. Functional connectivity of the cerebellar nuclei to thalamic and striatal regions of interest (ROIs) in patients with first-episode psychosis (FEP) compared with healthy control subjects (HCs)a
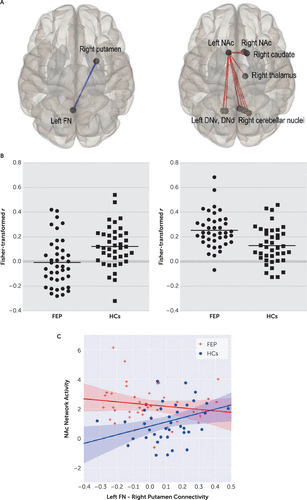
FIGURE 2. Functional connectivity of the cerebellar nuclei with striatal and thalamic regions in patients with first-episode psychosis compared with healthy control subjects (HCs)a
a Panel A shows patients with decreased functional connectivity between the left fastigial nucleus (FN) and right putamen compared with healthy control subjects. Patients exhibited an enhanced network activity linking the nucleus accumbens (NAc) with widespread regions (Panels A and B). The corresponding scatterplots are shown in Panel B. The correlations between the left FN and right putamen connectivity and the left NAc network activity in patients and HCs are shown in Panel C. HCs showed a positive correlation between the left FN and right putamen connectivity and the left NAc network activity (r=0.358, p=0.023), which was absent in the patient group (r=−0.152, p=0.349). DNd=dentate nucleus dorsal, DNv=dentate nucleus ventral, FEP=first-episode psychosis, FN=fastigial nucleus, NAc=nucleus accumbens.
At network level, we found a significantly increased intensity of left nucleus accumbens (NAc) network in patients, compared with HCs (p=0.035, FWE-corrected). This network comprised of individual connections of all cerebellar output nuclei, except for the left FN and IN, to the left NAc. Left NAc connections with the right NAc, caudate and thalamus were also included (Figure 2 right column). This network activity was negatively correlated with letter fluency performance that fell short of statistical significance among patients (r=−0.269, p=0.094).
HCs showed a positive correlation between the left FN and right putamen connectivity and the left NAc network activity (r=0.358, p=0.023), which was absent in patients (r=−0.152, p=0.349) (Figure 2C). Within patients, these connectivity results were not significantly associated with any of three syndrome scores. Individual symptoms associated with these results were ‘difficulty in abstract thinking’ (with left FN and right putamen connectivity: r=−0.388, p=0.013) and ‘excitement’ (with the NAc network activity: r=0.341, p=0.031).
Unique Functional Connectivity Analyses
Each ROI’s unique influence to the whole brain was examined by controlling for the contributions from other ROIs. As shown in Figure 3, patients exhibited decreased RSFC between the left IN and the right-sided homolog of Brodmann’s area (BA 45). By contrast, increased RSFC of the right IN with left inferior parietal cortex (BA 39, angular gyrus) and right premotor cortex (BA 6), and increased right DNv connectivity with left lateral frontopolar area (FP1, BA 10) were observed in patients. The unique connectivity increase was associated with disorganization symptoms (right IN connectivity with the left inferior parietal cortex: r=−0.462, p=0.003; right DNv with left lateral frontopolar area: r=0.338, p=0.033).
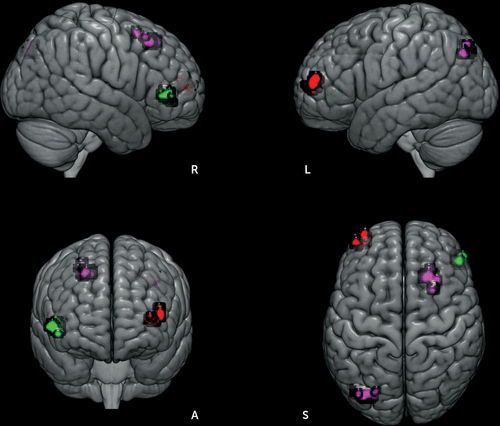
FIGURE 3. Unique functional connectivity analysis results among patients with first-episode psychosis compared with healthy control subjects (HCs)a
a Patients showed decreased left interpositus nucleus connectivity with the right-sided homolog of Brodmann’s area (BA 45) (green), compared with healthy control subjects. Patients also showed excessive right interpositus nucleus connectivity with the left inferior parietal cortex (BA 39, angular gyrus) and right premotor cortex (BA 6) (purple) as well as the right dentate nucleus ventral connectivity with the left lateral frontopolar area (BA 10) (red), compared with healthy control subjects. L=left, R=right.
DTI Findings
We found significantly increased axial diffusivity and an increase in RD values that fell short of statistical significance in the left SCP in patients (t=3.03, df=78, p=0.004; t=1.731, df=78, p=0.088), where both measures were highly positively correlated with each other (r=0.422, p=0.007). Hence, left SCP mean diffusivity values were significantly increased in patients (t=2.26, df=78, p=0.026). FA values for the left SCP were not different between groups (t=0.707, df=78, p=0.482). In addition, we found a level of increased right SCP FA values that nearly reached statistical significance among patients (t=1.745, df=78, p=0.085), which were positively correlated with current GAF scores (r=0.332, p=0.036). The means and standard deviations of DTI variables for three pairs of cerebellar peduncles were documented in Table S2 in the online supplement.
Volumetric Findings
Means and standard deviations for all volumetric variables were presented in Table S3 in the online supplement. Head-size-corrected (regional volume divided by intracranial volume) bilateral putamen (left: t=2.41, df=78, p=0.019; right: t=2.01, df=78, p=0.048), left NAc (t=1.78, df=78, p=0.079), left anterior cerebellar volumes (t=1.90, df=78, p=0.061) were significantly or marginally significantly decreased in patients, compared with HCs. Psychomotor poverty scores were positively correlated left putamen volume (r=0.349, p=0.027).
Correlations Among Neuroimaging Variables
RSFC abnormalities in patients were not correlated with any DTI or volumetric measures in patients. Left putamen volume was positively correlated with left anterior cerebellar volume in patients (r=0.542, p=0.0003), but negatively correlated with right SCP FA values (r=−0.371, p=0.018) and right IN unique connectivity with right premotor area (r=−0.321, p=0.044) in patients. These correlations are consistent with factor analytic results (for further details, see Table S4 in the online supplement). These correlations were not found in HCs.
In HCs, the left FN and right putamen connectivity was positively correlated with bilateral putamen (left: r=0.381, p=0.015; right: r=0.409, p=0.009) and left NAc (r=0.332, p=0.036) volumes. Left anterior cerebellar volume was positively correlated with right SCP FA values (r=0.368, p=0.019). Yet, these associations were not found in patients.
Discussion
Our results provided evidence for disordered RSFC between cerebellar output nuclei and striatal regions at the early stage of schizophrenia. Furthermore, we demonstrated that cerebellar output nuclei exert dysfunctional influences to fronto-parietal areas in patients, which is independent of striatal dysfunction. Finally, we highlighted that a normal structural-functional relationship is impaired in patients, by examining RSFC associations with DTI and volumetric measures.
Patients exhibited decreased RSFC between left FN and right putamen ROIs. The FN is critically involved in autonomic and oculomotor functions, that are well-known to be impaired in schizophrenia.23 In experimental animals, electrical stimulation of the FN increased blood flow in the dorsal striatum, sensorimotor and fronto-parietal areas, with a long-lasting neuroprotection effect against excitotoxic lesions of the striatum induced by a psychostimulant.24 In turn, the right putamen has been part of fronto-parietal cognitive network that is significantly underactivated in schizophrenia patients during executive function tasks including verbal fluency.25 This decrease of left FN and right putamen connectivity was associated with poor letter fluency performance, and also with lower GAF scores (both current and past year’s) in patients. This RSFC deficit may therefore represent a biomarker associated with executive dysfunction and poor outcome in patients.
Patients showed a widespread increase of the left NAc network. This hyperconnectivity also appeared to be dysfunctional, because letter fluency performance decreased (at a level that fell short of statistical significance), as the network activity increased. The conflicting hypo- and hyperconnectivity results are also commonly reported in both first-episode and chronic schizophrenia patients groups.26 Abnormally increased RSFC might reflect a compensatory, plasticity response to a primary pathophysiological process producing a disruption of topography of RSFC.27 Rather, we propose that our finding of both hypo- and hyperconnectivity results from different pathological sources. Our correlation and factor analysis results support our proposal, as they were unrelated to each other in patients (for further details, see Table S4 in the online supplement). These results have important implications for repetitive transcranial magnetic stimulation studies targeting the cerebellar vermis (and underlying the FN) using a facilitation protocol in schizophrenia.28 The mechanism of action in reducing symptom severity appears to be ameliorating functional connectivity decrease (e.g., left FN and right putamen connectivity as in our study) associated with cognitive and functional impairments. Then, a high level of excitement (e.g., agitation) would be a contraindication to such a treatment, as shown in our symptom correlation results.
We used a seed-to-voxel semipartial correlation analysis to investigate between-group differences in unique RSFC between our ROIs and the whole brain voxels. This semipartial correlation analysis, similar to multiple regression analysis, assesses a seed’s ‘unique’ part of the total variance at each voxel, relative to the residual, in the presence of signals from other seeds.29 Our results suggest that most if not all striatal-cortical RSFC abnormalities in patients are accounted by abnormal cerebellar influences, because no striatal seeds produced any significant between-group differences. It is particularly noteworthy that patients displayed significantly decreased unique RSFC between the left IN and right Broca’s area. It is well established that the IN, together with Broca’s area (BA 44/45), plays an important role in the timing of delay eye blink conditioning and in various timing tasks.5,30 Delay eye blink conditioning and timing functions are impaired in both schizophrenia patients and their first-degree relatives.31–33 The interplay between cerebellar and prefrontal areas appears to be critical for timing dysfunctions in schizophrenia.
We sought converging evidence from RSFC, DTI and volumetric investigations, which would be a distinct strength of our study. We did so, because there would be a possible compensatory increase of RSFC in case of gray-matter loss and/or impaired white-matter integrity.34 Two correlation results are of particular mention in this respect. Neither left anterior cerebellar nor right putamen volume was associated with the decrease of left FN and right putamen RSFC in patients. By contrast, this left FN and right putamen RSFC was positively correlated with bilateral putamen and left NAc volumes in HCs. The other important correlation is relating to the left NAc network activity which was increased in patients. Because left NAc volume was decreased at a level that fell short of statistical significance among patients, one would expect a significant negative correlation between left NAc volume and the strength of NAc network activity. However, it was not the case in our results. Taken together, these results indicated that normal structural-functional relationships are lost in patients. Consistent with this, studies investigating a structure-function relationship using DTI and resting-state fMRI have not found correspondence between the two measures in chronic schizophrenia patients.35 We suggest that multiple brain abnormalities from different origins do not converge even at the chronic stage of this illness.
A reduction in white-matter integrity in the left superior cerebellar peduncle (SCP) was found in patients, in terms of increased axial diffusivity, radial diffusivity (RD at level that fell short of statistical significance) and mean diffusivity. Increased axial diffusivity has been seen in an end state of axonal damage (i.e., when axon fragments are cleared), while increased RD has been related to demyelination.36 Although the exact meaning of this finding would need to be investigated in future studies, a number of previous studies have reported abnormalities that are specific to the SCP in chronic schizophrenia patients.37,38 The majority of these reported predominant left SCP abnormalities. Furthermore, adolescents at high risk for psychosis progressively exhibited reduced FA values in the SCP (left more than right) over 12 months.39 However, the middle cerebellar peduncle (MCP) which carries input fibers from the contralateral cerebral cortex to the cerebellar cortex via the pons was not significantly lowered in adolescents with first-admission or chronic schizophrenia patients.40,41 We also documented that the MCP was not disordered in our patients (for further details see Table S2 in the online supplement).
There are further issues to consider in interpreting our results. First, we did not perform statistical correction procedures for possible type I errors for our correlation analyses and between-group analyses for DTI and volumetric variables. When significant (or near-significant) between-group differences were found, we sought to find their cognitive, symptomatic and functioning correlates (84 comparisons). Thus, our correlational and DTI and volumetric between-group results must be interpreted with caution. Nonetheless, they may provide a basis for interpreting our neuroimaging data and generating new hypotheses. Second, our ROIs did not include cerebellar cortical areas for investigating cortico-ponto-cerebellar cortical connectivity (mediated by the MCP), because the most inferior edge of the cerebellar cortex was not covered in our fMRI scan for approximately 20% of participants (but the cerebellar nuclei were covered in all participants). We did not want to introduce a selection bias based on head-size. Moreover, our focus was on the influence of cerebellar output nuclei to the striatum and cerebral cortex, as available cerebellar DTI studies to date reported predominant abnormalities in the SCP (consistent with our DTI results, see Table S2 in the online supplement). Thirdly, special care was taken to minimize a possible signal contamination from neighboring ROIs. We could not completely rule out this possibility for cerebellar nuclei due to their spatial proximity. Nonetheless, our specific RSFC patterns did indicate that signals from different cerebellar nuclei were independent from each other, displaying different connectivity profiles. Finally, future studies for cerebellar functional connectivity may include timing tasks that are sensitive to cerebellar impairment (Lee et al., 2009, 2007).
In conclusion, our study provides evidence that RSFC between cerebellar output nuclei and striatal regions is disordered in patients at the early stage of schizophrenia. We found that dysfunctional cerebellar influence to the whole brain is above and beyond that of the striatum in patients. We further present comprehensive descriptions for abnormalities in cerebellar influence to striatal and cerebral cortical areas by examining RSFC, DTI, and volumetric data in the same group of first-episode psychosis patients. As such, our study provides a basis from which future investigations on cerebellar-striatal interaction abnormalities in schizophrenia are performed.
1 : A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 2010; 65:585–596Crossref, Medline, Google Scholar
2 : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
3 : Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull 2013; 39:387–395Crossref, Medline, Google Scholar
4 : The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 2010; 107:8452–8456Crossref, Medline, Google Scholar
5 : Consensus paper: current views on the role of cerebellar interpositus nucleus in movement control and emotion. Cerebellum 2013; 12:738–757Crossref, Medline, Google Scholar
6 : Cerebellar pathways to ventral midbrain and nigra. Exp Neurol 1976; 53:714–728Crossref, Medline, Google Scholar
7 : Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry 2011; 2:73Crossref, Medline, Google Scholar
8 : Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep 2015; 5:17275Crossref, Medline, Google Scholar
9 : Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci USA 2010; 107:4407–4411Crossref, Medline, Google Scholar
10 : The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci 2015; 370:370Crossref, Google Scholar
11 : Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum 2014; 13:386–410Medline, Google Scholar
12 : A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am J Psychiatry 2002; 159:227–237Crossref, Medline, Google Scholar
13 : Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2:125–141Crossref, Medline, Google Scholar
14 : Movement-related effects in fMRI time-series. Magn Reson Med 1996; 35:346–355Crossref, Medline, Google Scholar
15 : A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 2013; 76:183–201Crossref, Medline, Google Scholar
16 : Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014; 84:320–341Crossref, Medline, Google Scholar
17 : Functional connectivity-based parcellation of amygdala using self-organized mapping: a data driven approach. Hum Brain Mapp 2014; 35:1247–1260Crossref, Medline, Google Scholar
18 : Network-based statistic: identifying differences in brain networks. Neuroimage 2010; 53:1197–1207Crossref, Medline, Google Scholar
19 : Disentangling Brain Graphs: A Note on the Conflation of Network and Connectivity Analyses. Brain Connect 2016; 6:95–98Crossref, Medline, Google Scholar
20 : A probabilistic atlas of the cerebellar white matter. Neuroimage 2016; 124(Pt A):724–732Crossref, Medline, Google Scholar
21 : Development of a multilingual aphasia battery. Progress and problems. J Neurol Sci 1969; 9:39–48Crossref, Medline, Google Scholar
22 : Syndromes of schizophrenia and smooth-pursuit eye movement dysfunction. Psychiatry Res 2001; 101:11–21Crossref, Medline, Google Scholar
23 : Cerebellum and ocular motor control. Front Neurol 2011; 2:53Crossref, Medline, Google Scholar
24 : Intrinsic neurons of fastigial nucleus mediate neurogenic neuroprotection against excitotoxic and ischemic neuronal injury in rat. J Neurosci 1999; 19:4142–4154Crossref, Medline, Google Scholar
25 : Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 2009; 66:811–822Crossref, Medline, Google Scholar
26 : Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci 2015; 35:267–286Crossref, Medline, Google Scholar
27 : Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One 2011; 6:e22153Crossref, Medline, Google Scholar
28 : Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res 2010; 124:91–100Crossref, Medline, Google Scholar
29 : Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Mahwah, NJ, Erlbaum, 2013Crossref, Google Scholar
30 : fMRI identifies the right inferior frontal cortex as the brain region where time interval processing is altered by negative emotional arousal. Hum Brain Mapp 2015; 36:981–995Crossref, Medline, Google Scholar
31 : Impaired cerebellar-dependent eyeblink conditioning in first-degree relatives of individuals with schizophrenia. Schizophr Bull 2014; 40:1001–1010Crossref, Medline, Google Scholar
32 : Time perception and its neuropsychological correlates in patients with schizophrenia and in healthy volunteers. Psychiatry Res 2009; 166:174–183Crossref, Medline, Google Scholar
33 : Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn 2005; 58:109–118Crossref, Medline, Google Scholar
34 : The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. Neuroimage 2014; 102:118–127Crossref, Medline, Google Scholar
35 : Disruption of structure-function coupling in the schizophrenia connectome. Neuroimage Clin 2014; 4:779–787Crossref, Medline, Google Scholar
36 : Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 2006; 32:1090–1099Crossref, Medline, Google Scholar
37 : Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res 2008; 163:193–200Crossref, Medline, Google Scholar
38 : Abnormalities in myelination of the superior cerebellar peduncle in patients with schizophrenia and deficits in movement sequencing. Cerebellum 2014; 13:415–424Crossref, Medline, Google Scholar
39 : Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull 2014; 40:1204–1215Crossref, Medline, Google Scholar
40 : A diffusion tensor imaging study of middle and superior cerebellar peduncle in male patients with schizophrenia. Neurosci Lett 2003; 348:135–138Crossref, Medline, Google Scholar
41 : The optic radiation and the cerebellar peduncles in adolescents with first-admission schizophrenia--a diffusion tensor imaging study. J Neuroimaging 2014; 24:111–116Crossref, Medline, Google Scholar



