Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Review of Psychiatric Phenotypes and Management Considerations: A Report of the American Neuropsychiatric Association Committee on Research
Abstract
Objective:
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an autoimmune disorder characterized by prominent neuropsychiatric symptoms. Given the nature of its pathophysiology, psychiatrists tend to be one of the first clinicians encountering patients with the disease.
Methods:
In the present review of patients described in the literature with psychiatric symptoms, the authors aimed to characterize the psychiatric symptoms of the disease and its management in adults and adolescents as well as children (≤12 years old). A total of 544 patients fulfilled the inclusion criteria.
Results:
The authors found that 77% of patients with NMDAR encephalitis presented initially with psychiatric symptoms. These were mostly agitation (59%) and psychotic symptoms (in 54%, especially disorganized behavior and visual-auditory hallucinations), with agitation even more commonly being the presenting symptom in children (66%). Where psychotic symptoms were detailed, visual (64%) and auditory (59%) hallucinations were the most common, as well as persecutory delusions (73%). However, delusions were not clearly characterized in most cases. Catatonia was described in 42% of adult patients and 35% of children. Of the patients with documented exposure to antipsychotics, 33% were suspected to have an adverse drug reaction (notably, neuroleptic malignant syndrome in 22% of the cases).
Conclusions:
On the basis of these findings, it is important to consider anti-NMDAR encephalitis in the differential diagnosis of patients with an acute onset psychosis, especially in association with agitation, catatonia, or adverse response to antipsychotics. Furthermore, it is important to use antipsychotics with caution in patients with suspected or confirmed anti-NMDAR encephalitis.
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, first thought to be exclusively a paraneoplastic disorder, is an increasingly reported autoimmune disorder with a prominent neuropsychiatric presentation (1, 2). The clinical syndrome is characterized typically by a nonspecific, flu-like prodrome, in some cases followed by an abrupt onset of severe neuropsychiatric symptoms over the following 1–3 weeks (3). Predominant symptoms are cognitive impairments in memory and executive function, seizures, and behavioral symptoms such as impulsivity, disinhibition, or aggression (4). After this initial phase, patients then typically progress to develop movement disorders such as catatonia and dyskinesias, autonomic instability and hypoventilation, coma, or even death. Due to the predominance of the presenting neuropsychiatric symptoms, patients may be seen first by a psychiatrist.
The precise incidence of anti-NMDAR encephalitis is not established, although epidemiologic data suggest it is the second most common form of autoimmune encephalitis and is even more common than viral encephalitis in young people (4). The disease mainly affects young women: 37% are under the age of 18, and 81% of cases are female (4). The antibody implicated in the disorder was first described in 2007 (2). Since then, as the antibody test has become more widely available, the number of reported cases has rapidly expanded, with the largest reported series including 577 individuals (4). The pathophysiology involves antibody-mediated crosslinking and internalization of the GluN1 subunit of the NMDA receptor, which results in receptor hypofunction and altered synaptic plasticity (5). In roughly 40% of young women, a tumor is identified, typically a mature ovarian teratoma (4).
The diagnosis is suspected based on the characteristic clinical presentation and is confirmed with the detection of IgG anti-GlunN1 antibodies in the serum and ideally in the cerebrospinal fluid, along with supporting abnormalities on investigations, such as MRI, EEG and CSF analysis (6). Treatment centers on surgical removal of a tumor (if identified) and immunotherapies such as intravenous immunoglobulin, plasma exchange, corticosteroids, and rituximab. The prognosis can be favorable, with up to 75% of patients achieving full or nearly full recovery (7).
The management of neuropsychiatric symptoms is challenging. The condition is easily misdiagnosed (8), and there is often a delay of weeks between symptom onset and laboratory confirmation of the diagnosis (9). During this time, clinicians are left to prescribe symptomatic treatments, such as for seizure activity, catatonia, or perceptual disturbances. The use of antipsychotics in these patients, however, may worsen symptoms or cause neuroleptic malignant syndrome (10, 11); benzodiazepines, which may improve catatonia, may increase the risk of sedation in these patients who are already vulnerable to hypoventilation and decreased arousal (10). Adding complexity to the management of these patients is the likelihood that, as the diagnosis is suspected, certain patients may be undergoing major surgery (e.g., laparotomy), systemic immunotherapy (e.g., steroids, cyclophosphamide, rituximab, intravenous immunoglobulin), or plasma exchange. Although certain patients may be communicative and relatively cognitively intact, others may be mute, cognitively impaired, or even comatose. Throughout the course of their diagnostic workup, patients may reside on and transition between psychiatry wards or neurology wards, intensive care units, or emergency departments (12)—settings with very different physical environments and varying capacities for delivering behavioral interventions.
Improving the safety and quality of care provided to patients suffering from anti-NMDAR encephalitis may be possible with earlier recognition of the behavioral syndrome (9). The majority of reports of the disorder are published in the neurology literature and contain detailed descriptions of seizures and other traditional neurologic symptoms but may not go beyond the use of nonspecific phenomenological descriptors of behavioral symptoms, such as “agitation” or “psychosis” (5). Because patients may present first to psychiatrists, a more specific definition of the typical behavioral syndrome, if one exists, may facilitate earlier diagnosis and treatment. To further develop a descriptive psychopathology of anti-NMDAR encephalitis, we therefore sought to characterize the psychiatric phenotype and pharmacologic management of all such patients reported in the published literature.
Methods
We performed a PubMed and Embase search with the following key words: anti-NMDA OR NMDA OR anti-N-methyl-D-aspartate OR N-methyl-D-aspartate AND encephalitis. The search included manuscripts written in English, published through October 2016, and including human subjects. Only case reports and case series with documentation of serum or CSF anti-NMDAR antibodies as well as descriptions of the clinical presentation were included.
The patients had to have a documented clinical presentation of the acute to subacute (<3 months) onset of psychiatric symptoms in the setting of their disease. Studies of the prevalence of the antibodies in the setting of chronic neurologic or psychiatric disease were specifically excluded from analysis. In patients who had only psychiatric symptoms, the presence of CSF anti-NMDAR antibodies or a teratoma was required. Cases reported in more than one article were included only once.
All anti-NMDAR antibody tests were included for the purposes of analysis. A note was made of how the antibodies were measured for each article.
Articles were divided equally among four reviewers (RAS, MJC, JJC, IH), who used a common data extraction sheet. Each article was reviewed by one of the reviewers. The data extracted included the indication of the presence of delusions, perceptual disturbances (illusions or hallucinations), disorganized thought process, disorganized behavior, mood changes, anxiety, catatonia (e.g., mutism, echolalia, echopraxia), and agitation. Information regarding the presence or absence of anti-NMDAR antibodies in the CSF and the identification of a neoplasm was also noted. Data regarding treatment included the use of antiepileptic drugs (AEDs), including clobazam, GABAergic agents (clonazepam, diazepam, lorazepam, zolpidem, anesthetics), antipsychotics, ECT, and the documentation of any clear adverse reactions was noted.
Results
A total of 544 patients with anti-NMDAR encephalitis and a psychiatric-behavioral presentation were identified from 283 case reports and case series (Figure 1). Females constituted 75% of the study population, and the average age of all patients was 19.4 years (range, 2 months–84 years). Serum antibodies were noted to be positive in 63% of patients (no serum data documented, 29%; negative, 6%; positive but unclear whether CSF or serum, 2%), and 73% had a positive CSF (no CSF data documented, 25%; negative, 1%; positive but unclear whether CSF or serum, 2%). A tumor was identified in 24% of cases (no data, 22%; negative, 53%). Seventy-seven percent of the sample had either CSF anti-NMDAR antibodies or a teratoma.
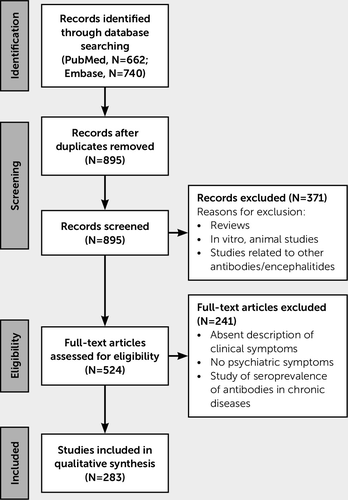
FIGURE 1. Selection of articles for inclusion in the analysis
Of the 283 articles, 94 either described the way of measuring the antibodies or referenced a lab or university hospital. The remaining articles only noted antibody positivity.
Psychiatric Phenotype
Of the 515 case subjects for which a clear chronology of symptoms was described, 76.7% (N=395) presented with psychiatric symptoms. In the whole cohort of 544 patients, the most common symptom described was agitation (59%, N=322) (Figure 2), although the reports lack a consistent phenomenological or operational definition of agitation. Self-injurious behavior was highlighted in 3.6% (N=20) of the whole cohort. Psychotic symptoms were the second most common symptom (54%, N=294) (Figure 2).
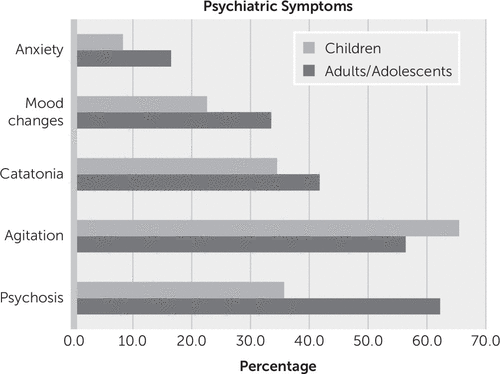
FIGURE 2. Psychiatric symptoms in adults/adolescents (>12 years old) and children (≤12 years old) with anti-N-methyl-D-aspartate receptor encephalitis
Catatonia was a commonly reported phenomenon in both adults (42%, N=157) and children (35%, N=58). Although in some cases the authors explicitly identified a catatonia syndrome, in many others the signs and symptoms of catatonia were described but not explicitly identified as such by the authors. For example, symptoms of mutism, stupor or excessive motor activity, or echopraxia or echolalia were described in several reports (13–25). In the whole cohort, mutism was specifically listed in 9.8% of cases (N=53), whereas echopraxia or echolalia were listed in 2.3% (N=12).
Major mood symptoms, such as depression or mania, were reported in 30.0% (N=164) of the cases, and anxiety in 13.9% (N=76). It was not possible to ascertain the extent to which the commonly reported symptom of agitation may have included behaviors driven by mood states like mania, by anxiety, by psychosis, or by catatonia.
A difference between adults/adolescents and children (≤12 years old) was noted: children were more likely to present with agitation and less likely to describe psychotic symptoms (Figure 2).
In an analysis of the 252 cases with detailed descriptions of psychotic symptoms, delusions were reported in 32.9% (N=83); where the nature of the delusions was described, they were persecutory in 73% (N=16/22). Perceptual disturbances, such as hallucinations or illusions, were reported in 77% (N=176) of cases; where the sensory modality of the hallucinations was described, they were visual in 64% (N=51/80), auditory in 59% (N=47), olfactory in 3% (N=2), and tactile in 3% (N=2). Disorganized thinking, characterized as tangentiality, perseveration, pressured or incoherent speech, or thought blocking, was documented in 13% (N=33) of cases. Finally, although disorganized behavior was the most common form of psychotic symptom, described in 70% (N=177) of cases, the reports lacked a consistent phenomenological or operational definition of disorganized behavior (Table 1).
| Symptom | Adult/Adolescent (N=203) | Children (N=49) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Delusions | 78 | 38.4 | 5 | 10.2 |
| Perceptual disturbances | 153 | 75.4 | 23 | 47.0 |
| Disorganized thinking | 28 | 13.8 | 5 | 10.2 |
| Disorganized behavior | 134 | 66.0 | 43 | 87.7 |
TABLE 1. Frequency of psychotic symptoms in patients with anti-N-methyl-D-aspartate receptor encephalitis in articles with specific symptom descriptionsa
Management Considerations
A total of 250 patients had information available about the use of medications other than immunotherapy during the management of symptoms. Medication categories were AEDs, 62%; GABAergic drugs, including anesthetics, 45%; and antipsychotics, 44%. A total of 12 patients received ECT. Other relevant medications included sleep aids such as melatonin, clonidine, memantine, antidepressants (selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, trazodone, tricyclics), lithium, dopaminergic agents (bromocriptine, amantadine), trihexyphenidyl, and tetrabenazine. One patient received vagus nerve stimulation (26), and one was treated with a ketogenic diet (27).
Neuroleptic malignant syndrome was diagnosed or suspected in 25 (22%) of the patients documented to have been exposed to an antipsychotic. Information on medication exposure was limited (haloperidol, N=3; olanzapine, N=2; risperidone, N=1). Extrapyramidal symptoms secondary to antipsychotic exposure were suspected in 12 (11%) patients. In these cases, medication information was limited (haloperidol, N=4; risperidone, N=3; quetiapine, N=1).
Discussion
This review attempts to characterize the psychiatric phenotype of anti-NMDAR encephalitis. Despite the fact that the psychiatric symptomatology has been prominently highlighted since the first description of this disorder in 2007 (2), in our review the descriptive psychopathology often was either absent or lumped into psychiatric or neuropsychiatric categories. In contrast, more traditional neurologic symptoms were better characterized (e.g., seizures, memory impairments, coma). Notwithstanding this limitation, our review highlights the important fact that over 75% of patients with psychiatric symptoms had these symptoms at disease onset.
Subset analyses revealed three important more specific findings. First, various forms of psychotic symptoms are prominent features of the disorder. This finding is not unexpected, as the blockade of NMDA receptors with phenylcyclohexylpiperidine or ketamine can be associated with psychotic symptoms (28, 29). The glutamate hypothesis in schizophrenia emphasizes the central role of NMDA receptors in the pathophysiology of the disease both with its explanation of the negative symptoms and its effect on mesolimbic dopaminergic systems in the generation of positive symptoms (30). When compared with adults, children with anti-NMDAR encephalitis tend to have less prominent psychiatric symptoms and more prominent neurologic symptoms (31). The onset of psychotic symptoms in idiopathic psychiatric disorders before the age of 13 is extremely rare (32), and the predominant phenotype in the pediatric patients with anti-NMDAR encephalitis is that of agitation.
Second, neuroleptic sensitivity is a relatively common feature of the disorder (22% of patients), a finding that carries therapeutic implications. This finding is also confirmed by a study of a large French cohort (11), which described 21 patients with anti-NMDAR antibody encephalitis with neuroleptic sensitivity to both first- and second-generation antipsychotics. Antipsychotic use should be considered carefully in this population because of the risk of precipitating autonomic instability, particularly when catatonic features are present. There are insufficient data to recommend any particular therapeutic approach for the treatment of psychosis in patients with anti-NMDAR encephalitis, although one study has advocated the use of a low potency antipsychotic such as quetiapine in younger patients (33). Other than immunotherapeutic agents, a host of other psychotropic medications are often prescribed given the different symptoms associated with the disease, such as seizures, insomnia, dysautonomia, and movement disorders. Nonpharmacologic options such as ECT have shown some success in the treatment of catatonic symptoms (34). There is no evidence that long-term treatment with antipsychotic medications prevents relapse among persons with psychotic symptoms due to anti-NMDAR encephalitis. Accordingly, antipsychotic medications and other symptomatic treatments, when used, should be discontinued following definitive treatment of the anti-NMDAR encephalitis itself.
Finally, the most common psychiatric symptoms described at the onset of anti-NMDAR encephalitis may not be distinct from those seen in schizophrenia. Anti-NMDAR antibody encephalitis is a rare disorder relative to schizophrenia, which has an estimated annual incidence of 0.85 per million among children (35). Movement disorders, including catatonia, are common in both disorders. Furthermore, the nature of the perceptual disturbances and delusional thinking may also be similar in both disorders. Data on patients with new onset psychosis not due to autoimmune encephalitis show relatively higher rates of auditory hallucinations (74%), followed by visual (39%), tactile (28%) and olfactory (19%) hallucinations (36). Although certain red flags for encephalitis may exist, it may not always be easy for clinicians to distinguish between the two disorders based on clinical presentation alone. Herken and Prüss (37) identified several red and “yellow” flags that can aid in expediting the diagnosis and show how some may have already been documented in the chart by the psychiatric team. Patients with isolated psychiatric symptomatology in the setting of anti-NMDAR encephalitis have been described by Kayser et al. (38). In their analysis of 571 patients, five (0.9%) had isolated psychiatric episodes with delusions as part of their initial presentation; all of them had neuroimaging done and were found to have an abnormal MRI. Lejuste et al. (11) analyzed 111 patients using strict criteria for positive antibodies in the CSF and found that 39% were first under the care of psychiatric departments; 47% were ultimately transferred due to suspicion of neuroleptic malignant syndrome.
Moreover, if clinicians obtain antibody testing only in those individuals with atypical features or wait for disease progression and the development of neurologic and autonomic symptoms, then the diagnosis of anti-NMDAR encephalitis can be missed in some cases; cases that are identified will be caught at a later stage of the illness, with an associated worse prognosis. Therefore, we encourage antibody screening in any patient with acute onset psychotic symptoms or agitation, especially if these symptoms are accompanied by catatonia, altered consciousness, or a flu-like prodrome. Further investigation is needed to determine whether universal antibody screening in all persons with new onset psychosis is indicated and, if so, whether such screening is cost effective.
The most commonly used screening test involves testing serum on a cell-based assay, with some evidence that live cell-based assays are more sensitive at detecting these low levels of antibody (39) than the more widely available fixed cell-based assays. These live cell-based assays may therefore be particularly useful for testing patients in psychiatric settings where the antibody levels, at the onset of illness, may be at a low level. However, they are more technically difficult to perform and are currently only undertaken at a few labs around the world. In the case of a negative serum test and a compelling clinical history (i.e., adverse response to antipsychotics, emergence of other suggestive symptoms), we recommend retesting for antibodies after 4 weeks. In the case of a positive serum antibody test, we recommend further investigations. There are some studies that show anti-NMDAR antibodies at low levels in otherwise healthy individuals. Therefore, a positive serum antibody test with a clinically indistinct presentation does not necessarily indicate the need for immune therapy. Further investigation with EEG, MRI, and lumbar puncture will provide further information to support treatment decisions. This is in line with existing clinical consensus guidelines for probable anti-NMDAR encephalitis (6), which require acute onset symptoms from at least four of the following: psychiatric, speech dysfunction, seizures, movement disorders, decreased level of consciousness, or autonomic dysfunction. Lab results require either an abnormal EEG or abnormal CSF (pleocytosis or oligoclonal bands). Three of the six groups of clinical symptoms and the presence of a teratoma also qualify for probable anti-NMDAR encephalitis. For a definite diagnosis, one group of symptoms and the presence of IgG anti-GluN1 antibodies in CSF are needed; if serum testing only is included, confirmatory live neurons or tissue immunohistochemistry is required.
Given that CSF testing is a more direct measure of antibodies in the CNS, it should therefore be the recommended investigation for establishing whether a patient with a suspicious neuropsychiatric presentation has anti-NMDAR encephalitis. This is particularly helpful if the patient has a low level of serum antibodies, where it is uncertain whether the antibodies are physiologically relevant. However the absence of antibodies in the CSF is not a definitive exclusion of a diagnosis of anti-NMDAR encephalitis; there is some evidence that the brain may in fact act as an immunoprecipitator to low levels of antibodies, such that they cannot be detected (40).
Limitations
This review has several limitations. The review is at risk of selection bias given its nonsystematic nature. The criteria used for including cases were less strict than other studies (11); 27% of the cohort had no clear documentation of CSF antibodies, and only a third of the articles included a clear reference of how or where the antibody testing was made. As a result, we cannot exclude the possibility that false positive cases have been included.
In addition, because the symptoms documented in the articles were not systematically collected, it is difficult to ascertain whether a symptom was in fact absent if it was not specifically mentioned. In particular, even when catatonic symptoms were described (e.g., mutism, posturing, echopraxia, stereotypies), the syndrome-level formulation of catatonia was often not made, consistent with data showing underrecognition and low knowledge of the syndrome (41, 42). Subthreshold catatonic symptoms (fewer than three of 12 symptoms) were seen in many cases, leading us to hypothesize that our methods underestimate the real prevalence of catatonia in anti-NMDAR encephalitis. Several studies also did not report the medications used in the symptomatic treatment of the disease and often only highlighted the immune treatments. Additionally, it was not clear to what extent the use of immune treatments, such as steroids and AEDs, may have iatrogenically influenced the psychiatric phenotype.
1 : Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol 2005; 58:594–604Crossref, Medline, Google Scholar
2 : Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61:25–36Crossref, Medline, Google Scholar
3 : Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7:1091–1098Crossref, Medline, Google Scholar
4 : Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165Crossref, Medline, Google Scholar
5 : Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010; 30:5866–5875Crossref, Medline, Google Scholar
6 : A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15:391–404Crossref, Medline, Google Scholar
7 : Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74Crossref, Medline, Google Scholar
8 : Psychiatric manifestations of anti-NMDA receptor encephalitis: neurobiological underpinnings and differential diagnostic implications. Psychosomatics 2014; 55:37–44Crossref, Medline, Google Scholar
9 : Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol 2011; 231:86–91Crossref, Medline, Google Scholar
10 : A multidisciplinary approach to the treatment of anti-NMDA-receptor antibody encephalitis: a case and review of the literature. J Neuropsychiatry Clin Neurosci 2012; 24:247–254Link, Google Scholar
11 : Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm 2016; 3:e280Crossref, Medline, Google Scholar
12 : Anti-NMDA receptor encephalitis: diagnosis, psychiatric presentation, and treatment. Am J Psychiatry 2011; 168:245–251Crossref, Medline, Google Scholar
13 : Bi-insular cortical involvement in anti-NMDA-receptor encephalitis - a case report. BMC Neurol 2016; 16:130Crossref, Medline, Google Scholar
14 : Clinical features, therapeutic response, and follow-up in pediatric anti-N-methyl-D-aspartate receptor encephalitis: experience from a tertiary care university hospital in India. Neuropediatrics 2016; 47:24–32Crossref, Medline, Google Scholar
15 : Could coenzyme Q10 supplementation have a role in the treatment of anti-NMDA receptor encephalitis? Acta Neurol Belg 2015; 115:85–86Crossref, Medline, Google Scholar
16 : Anti-NMDA-receptor antibody encephalitis in infants. Epilepsy Behav Case Rep 2015; 4:99–101Crossref, Medline, Google Scholar
17 : Uncommon organic psychosis: An adolescent case of Anti-N-Methyl-D-Aspartate receptor encephalitis. Dusunen Adam 2015; 28:171–174Crossref, Google Scholar
18 : Clinical and imaging characteristics of 16 patients with autoimmune neuronal synaptic encephalitis. Neurol India 2015; 63:687–696Crossref, Medline, Google Scholar
19 : Paraneoplastic neurological disorders in children with benign ovarian tumors. Brain Dev 2014; 36:248–253Crossref, Medline, Google Scholar
20 : Pediatric anti-N-methyl-D-aspartate (NMDA) receptor encephalitis: experience of a tertiary care teaching center from north India. J Child Neurol 2014; 29:1453–1459Crossref, Medline, Google Scholar
21 : Rituximab ameliorates anti-N-methyl-D-aspartate receptor encephalitis by removal of short-lived plasmablasts. J Neuroimmunol 2013; 265:128–130Crossref, Medline, Google Scholar
22 : Anti-N-methyl-D-aspartate (NMDA) receptor encephalitis in a young Lebanese girl. J Child Neurol 2013; 28:1222–1225Crossref, Medline, Google Scholar
23 : Early consideration of anti-NMDAR encephalitis in unexplained encephalopathy. Hong Kong Med J 2013; 19:362–364Crossref, Medline, Google Scholar
24 : Limbic encephalitis in a boy with N-methyl-D-aspartate receptor antibodies. J Pediatr 2012; 160:1060Crossref, Medline, Google Scholar
25 : Anti-N-methyl-D-aspartate receptor encephalitis with acute disseminated encephalomyelitis-like MRI features. Eur J Neurol 2012; 19:e16–e17Crossref, Medline, Google Scholar
26 : VNS terminating refractory nonconvulsive SE secondary to anti-NMDA encephalitis: A case report. Epilepsy Behav Case Rep 2015; 3:39–42Crossref, Medline, Google Scholar
27 : Refractory status epilepticus from NMDA receptor encephalitis successfully treated with an adjunctive ketogenic diet. Ann Indian Acad Neurol 2015; 18:256–257Crossref, Medline, Google Scholar
28 : Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 2007; 78:69–108Crossref, Medline, Google Scholar
29 : Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17:2921–2927Crossref, Medline, Google Scholar
30 : The glutamate hypothesis: a pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry 2014; 47:121–130Crossref, Medline, Google Scholar
31 : Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013; 162:850–856.e2Crossref, Medline, Google Scholar
32 : Psychosis in children and youth: focus on early-onset schizophrenia. Pediatr Rev 2013; 34:296–305, quiz 305–306Crossref, Medline, Google Scholar
33 : Agitation management in pediatric males with anti-N-methyl-D-aspartate receptor encephalitis. J Child Adolesc Psychopharmacol 2016; 26:939–943Crossref, Medline, Google Scholar
34 : Electroconvulsive therapy in anti-N-methyl-D-aspartate receptor encephalitis: a case report and review of the literature. J ECT 2016; 32:225–229Crossref, Medline, Google Scholar
35 : N-methyl-D-aspartate receptor antibody-mediated neurological disease: results of a UK-based surveillance study in children. Arch Dis Child 2015; 100:521–526Crossref, Medline, Google Scholar
36 : Auditory and non-auditory hallucinations in first-episode psychosis: Differential associations with diverse clinical features. Psychiatry Res 2017; 254:268–274Crossref, Medline, Google Scholar
37 : Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry 2017; 8:25Crossref, Medline, Google Scholar
38 : Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 2013; 70:1133–1139Crossref, Medline, Google Scholar
39 : Cell- and single molecule-based methods to detect anti-N-methyl-D-aspartate receptor autoantibodies in patients with first-episode psychosis from the OPTiMiSE Project. Biol Psychiatry 2017; 82:766–772Crossref, Medline, Google Scholar
40 : The brain as immunoprecipitator of serum autoantibodies against N-methyl-D-aspartate receptor subunit NR1. Ann Neurol 2016; 79:144–151Crossref, Medline, Google Scholar
41 : Catatonia: disappeared or under-diagnosed? Psychopathology 2005; 38:3–8Crossref, Medline, Google Scholar
42 : Catatonia education: needs assessment and brief online intervention. Acad Psychiatry 2017; 41:360–363Crossref, Medline, Google Scholar



