Deficits in Emotion Recognition as Markers of Frontal Behavioral Dysfunction in Amyotrophic Lateral Sclerosis
Abstract
Objective:
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease with prominent motor symptoms. Patients with ALS may also manifest frontal behavior symptoms and cognitive decline, including impairment in facial emotion recognition. The authors aimed to investigate whether deficits in emotion recognition were associated with frontal behavior symptoms in ALS.
Methods:
Participants were patients with probable or definite sporadic ALS (N=21; male:female ratio, 11:10; median age, 62 years; median disease duration, 3 years) and age-matched and education-matched healthy control subjects (N=25; male:female ratio, 14:11; median age, 61 years). The Facial Emotion Recognition Test (FERT) was administered to all participants. Patients with ALS were assessed using the Cambridge Behavior Inventory-Revised and were classified into two groups according to the presence of frontal behavioral symptoms: ALS with no behavioral symptom (ALSns; N=9) and ALS with at least one behavioral symptom (ALSbs; N=12).
Results:
Apathy and mood symptoms were the most frequent neuropsychiatric symptoms in the patient group. Patients with ALS performed worse than control subjects in the recognition of sadness (p<0.004). There were no differences between control subjects and patients in the ALSns group in all FERT scores, but the ALSbs group had lower performance than control subjects in sadness (p<0.003).
Conclusions:
Emotion recognition deficit may be a marker of frontal behavior in ALS.
Besides prominent motor impairment, patients with amyotrophic lateral sclerosis (ALS) manifest cognitive and behavioral symptoms.1,2 Although executive impairment is the most common cognitive dysfunction in ALS,3 social cognition deficits have also been reported,1,4 with decline in abilities such as theory of mind and emotional processing.1,2 In this latter regard, deficits in recognition of facial emotion expressions are present in ALS.1,5–10 Patients with ALS also exhibit behavioral symptoms such as apathy, loss of empathy, and personality changes,11 even at early stages of the disease and before motor symptoms.12 Cognitive and behavioral symptoms in ALS show great overlap with the presentation of behavioral variant frontotemporal dementia (bvFTD).
However, it is not clear whether emotion recognition deficits in ALS occur in the absence of behavioral changes, as some of the previous studies in the field did not assess frontal behavioral symptoms with specific tools.6,9,13 The issue of whether emotion recognition deficits can occur independently of frontal behavioral syndrome in ALS remains open. Therefore, the aim of this exploratory study was to investigate the possible association between recognition of facial emotions and frontal behavioral symptoms (e.g., abnormal eating habits, apathy, stereotypical and motor behaviors, and other abnormal behaviors) in ALS. Taking into account that both emotion recognition and behavioral control rely on the integrity of prefrontal cortex, we hypothesized that deficits in the recognition of facial emotional expressions would be associated with frontal symptoms in ALS.
Methods
This study was conducted at the University Hospital of the Federal University of Minas Gerais (Belo Horizonte, Brazil). The Local Ethics Committee approved the study, and all participants provided written informed consent.
Two groups of participants were enrolled: a consecutive series of patients diagnosed with probable or definite sporadic ALS (N=21; male:female ratio, 11:10; median age, 62 years), according to Awaji’s criteria;14 and healthy control subjects (N=25; male:female ratio, 14:11 female; median age, 61 years), matched with the ALS group on age, sex, and education level. The majority of patients (N=18/21) had spinal presentation. Demographic characteristics and clinical data for the study participants are summarized in Table 1.
| Characteristic | Control Subjects (N=25) | Amyotrophic Lateral Sclerosis (ALS) (N=21) | Amyotrophic Lateral Sclerosis Without Behavioral Symptoms (ALSns) (N=9) | Amyotrophic Lateral Sclerosis With Behavioral Symptoms (ALSbs) (N=12) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | P25-P75a | Median | P25-P75a | (ALS versus controls) pb | r | Median | P25-P75a | (ALSns versus controls) pb | r | Median | P25-P75a | (ALSbs versus controls) pb | r | |
| Age (years) | 61 | 54–65 | 62 | 56–66.5 | 0.740 | 0.05 | 64 | 49–66.5 | 0.969 | 0.10 | 61 | (58–66) | 0.666 | 0.07 |
| Education (years) | 5 | 4–11 | 5 | 1–3.5 | 0.787 | 0.04 | 6 | 4–9.5 | 0.848 | 0.03 | 5 | (4–10) | 0.597 | 0.09 |
| Sex (male/female) | 14/11 | NA | 14/11 | NA | 0.806c | NA | 5/4 | NA | 0.982c | NA | 6/6 | NA | 0.732c | NA |
| Disease duration (years) | NA | NA | 3 | 1–3.5 | NA | NA | 3 | 1–3.5 | NA | NA | 3 | (1–4) | NA | NA |
| Hospital Anxiety and Depression Scale | ||||||||||||||
| Anxiety | 4 | 2–6 | 8* | 4–13* | 0.002 | 0.47 | 6 | 4–12 | 0.066 | 0.32 | 8* | 5–13* | 0.001 | 0.55 |
| Depression | 2 | 1–5 | 6* | 4–10* | 0.002 | 0.46 | 6 | 3–11 | 0.055 | 0.34 | 7* | 4–11* | 0.003 | 0.48 |
| Mini-Mental Status Examination (score out of 30) | 26 | 25–28 | 24 | 20–27 | 0.018 | 0.35 | 25 | 23–27 | 0.130 | 0.27 | 23 | 18–28 | 0.030 | 0.36 |
| Fluency Letter S | NA | NA | 7 | 5–9 | NA | NA | 7 | 6–10 | NA | NA | 6 | 1–9 | NA | NA |
| Facial Emotion Recognition test | ||||||||||||||
| Total score (score out 35) | 23 | 21–26 | 21 | 17–26 | 0.147 | 0.21 | 23 | 17–28 | 0.730 | 0.06 | 19 | 17–26 | 0.066 | 0.30 |
| Happiness (score out 5) | 5 | 5–5 | 5 | 5–5 | 0.571 | 0.08 | 5 | 5–5 | 0.848 | 0.06 | 5 | 5–5 | 0.761 | 0.09 |
| Surprise (score out 5) | 4 | 2–4 | 3 | 2–4 | 0.640 | 0.07 | 4 | 2–5 | 0.908 | 0.02 | 3 | 2–5 | 0.597 | 0.09 |
| Disgust (score out 5) | 3 | 3–4 | 4 | 3–5 | 0.255 | 0.17 | 4 | 3–5 | 0.419 | 0.15 | 4 | 3–5 | 0.378 | 0.16 |
| Fear (score out 5) | 2 | 1–3 | 2 | 1–3 | 0.751 | 0.05 | 2 | 1–4 | 0.565 | 0.10 | 2 | 1–3 | 1.0 | 0.01 |
| Anger (score out 5) | 3 | 3–4 | 2 | 1–4 | 0.037 | 0.31 | 3 | 2–4 | 0.442 | 0.14 | 2 | 1–3 | 0.021 | 0.39 |
| Sadness (score out 5) | 3 | 3–4 | 2* | 1–3* | 0.004 | 0.43 | 3 | 2–4 | 0.140 | 0.27 | 2* | 1–3* | 0.003 | 0.49 |
| Neutral (score out 5) | 4 | 3–5 | 3 | 1–4 | 0.170 | 0.20 | 4 | 2–5 | 0.848 | 0.04 | 2 | 1–4 | 0.077 | 0.30 |
TABLE 1. Demographic, clinical, and neuropsychological data for patients and age-matched control subjects
Patients with ALS were evaluated with a standardized clinical protocol described elsewhere.15 We did not include patients who used noninvasive ventilation. Patients with severe psychiatric disorder (e.g., past diagnosis of bipolar disorder or schizophrenia) or with neurological disease other than ALS (e.g., past history of stroke, epilepsy) were not included.
Control subjects were recruited from the community on a voluntary basis. Control subjects were not included if they presented any of the following criteria: history of neurological or psychiatric disorders, or cognitive complaints. We did not include control subjects who scored below norms on the Mini-Mental Status Exam (MMSE).16
All participants underwent a brief examination that included the MMSE and the Facial Emotion Recognition Test (FERT) derived from the Social and Emotional Assessment,17 and the Hospital Anxiety and Depression (HAD) scale. The HAD provides separate subscales for anxiety (HAD-A) and depression (HAD-D). A score above 8 in each subscale indicates depression or anxiety.18 The FERT is composed of a panel of 35 pictures from Ekman’s portfolio,17 with seven different emotions (happiness, sadness, fear, disgust, surprise, anger, and neutral) presented five times each, as described elsewhere.17 Pictures are shown on a screen in a pseudorandomized order. It is important to note that labels of emotions (happiness, sadness, fear, disgust, surprise, anger, and neutral) are presented during the entire task to avoid impaired performance due to memory disorder. Participants pointed or verbally indicated their answers, which were recorded by the investigator. Patients with ALS also underwent a verbal fluency test (letter S in 1 minute) and the Portuguese version of the Cambridge Behavior Inventory-Revised (CBI-R).19 The CBI-R assesses 10 domains including functional abilities and cognitive, behavioral, and psychiatric symptoms. Here we considered the four domains that identify bvFTD symptomatology (abnormal eating habits, apathy, stereotypical and motor behaviors, and abnormal challenging behaviors). Any particular behavior is rated on a scale from 0 (no impairment) to 4 (constant occurrence). Patients were then classified in terms of severity of impairment according to CBI-R score on each subscale: 0%−25% was considered as mild, 26%−50% as moderate, 51%−75% as severe, and more than 75% as very severe.20 We classified patients with ALS according to the presence of moderate to very severe bvFTD symptoms (Figure 1A). Two subgroups were then established: one subgroup without any bvFTD feature (ALS without behavioral symptoms [ALSns]; N=9 [43%]) and ALS patients with at least one behavioral feature of bvFTD (ALS behavioral symptom [ALSbs]; N=12 [57%]). It is noteworthy that two patients (9%) in the ALSbs group had impairment in three of these domains, meeting the criteria for bvFTD (Figure 1A).
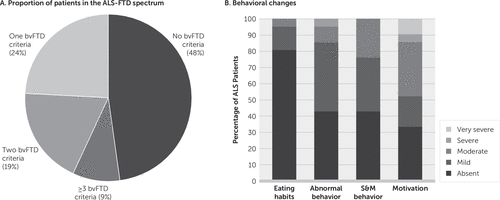
FIGURE 1. Behavioral changes among patients with amyotrophic lateral sclerosis (ALS)a
a Panel A shows the proportion of patients in the ALS-frontotemporal dementia (FTD) spectrum. Panel B shows the behavioral symptoms measured by the Cambridge Behavioral Inventory Revised in patients with ALS. bvFTD=behavioral variant frontotemporal dementia, S&M behavior=Stereotypical and motor abnormal behavior
Statistical Analyses
Descriptive statistics were used to characterize the sample. The normality assumption was investigated with the Kolmogorov-Smirnov test. The statistical assumption of normality was refuted. Accordingly, nonparametric tests were adopted. Group comparisons were performed in two steps. First, we used the Mann-Whitney U test to compare continuous variables between healthy control subjects and ALS (total group). Second, we used the Kruskal-Wallis test to compare continuous variables between the three study groups (healthy control, ALSns, and ALSbs); when pertinent, the Mann-Whitney U test was applied to perform 2×2 group comparisons. The chi-square test was used for comparing categorical variables between groups. After applying the Bonferroni correction, the p value was set at 0.005. Effect size was calculated with Pearson’s r. Correlations between variables were calculated using Spearman’s correlation test with Bonferroni correction. We used SPSS (Version 22; IBM, Armonk, N.Y.) for all analyses.
Results
There was no difference between the three study groups (healthy control, ALSns, and ALSbs) on sociodemographic variables (age, educational level, and sex distribution). The ALSns and ALSbs groups did not differ on disease duration.
Most of the ALS patients exhibited behavioral impairments according to CBI-R. Apathy was the most frequent behavioral disorder (48% of patients), with moderate to very severe intensity in all of them (Figure 1B).
Patients had higher scores than healthy control subjects on HAD-A and HAD-D scales (Table 1). Moreover, nine patients (9/21, 43%) scored above cut-off on the anxiety measure, and seven patients (7/21, 33%) scored above cut-off on the depression measure, indicating clinically relevant symptoms. Four patients in the ALSbs group had both depression and apathy, and four patients (including one patient with ALS-bvFTD) had both anxiety and apathy. Two ALSbs patients (including one patient with ALS-bvFTD) had depression, anxiety, and apathy.
Compared with control subjects, patients with ALS had lower performance on cognitive screening measures in the MMSE (p<0.02, r=0.35) (Table 1). Because of motor or speech impairment, 11 out of 21 patients (52%) did not complete the entire MMSE. When these patients with incomplete MMSE were excluded, there was no difference on the MMSE between the ALS and the control groups.
Patients (total group) did not differ from control subjects on the total score on the FERT and on all Ekman Faces Test categories, except sadness (p<0.004, r=0.43), where patients performed worse than controls (Table 1).
There was no difference between the control and ALSns groups on all FERT scores, whereas the ALSbs group had lower performance than the control group on sadness measures (p<0.003, r=0.49). We reran comparisons between control subjects and patients with ALSbs without the ALS-bvFTD group (N=2), and the same difference on the recognition of sadness fell just short of statistical significance (p<0.02). There was no difference between ALS subgroups (ALSbs versus ALSns) for all demographical, clinical, and cognitive measures, including FERT (Table 1).
No correlation was found between FERT and MMSE in the ALS group. Similarly, there was no correlation between FERT and verbal fluency. There was no statistically significant correlation between FERT (total score) and HAD-A and HAD-D scales.
Discussion
This study investigated the potential association between emotion recognition and behavioral symptoms in ALS. Apathy was the most prominent neuropsychiatric syndrome, which is in line with previous studies.11 Lack of motivation, changes in eating habits, abnormal behavior, and stereotypical and motor behavior are observed in bvFTD and are considered as core diagnostic criteria for this condition.21 In our sample, 57% of the patient group had at least one of these domains affected, and two patients (9%) had impairment in three of these domains, thus fulfilling criteria for bvFTD. This finding is in agreement with previous reports of estimated prevalence of bvFTD in up to 15% of patients.2,4 These findings support the concept of frontotemporal spectrum disorder in ALS.4
Depressive symptoms were also frequent, as 33% of patients had clinically relevant depressive symptoms according to HAD-D. Depressive symptoms may be observed in bvFTD22 and are commonly observed in ALS.23–25 Here we found that depressive or anxiety symptoms may overlap with apathy and other bvFTD features in ALS. Apathy, challenging behavior, and other neuropsychiatric features co-occur in frontotemporal lobar degeneration syndromes.26 It is unclear why there is association between depression, anxiety and bvFTD features in ALS. Further research is warranted to clarify this question.
Emotion recognition correlated neither with MMSE nor with an executive task (verbal fluency). Actually, there was no correlation between FERT and scores on psychiatric scales for anxiety (HAD-A) and depression (HAD-D). Social cognition impairment in ALS was not associated with depressive symptoms in a recent meta-analysis.1 Moreover, emotion recognition correlated neither with MMSE nor with an executive task (verbal fluency). These findings suggest that emotion recognition impairment in ALS does not reflect either mood disorders or deficits in general cognition/executive functions. This pattern is similar to the one seen in bvFTD but not in other neurodegenerative diseases such as Alzheimer’s disease, in which social cognition decline is mediated by executive dysfunction.27
ALS (total group) performed worse than controls in recognition of sadness. Functional neuroimaging studies in healthy subjects found that the processing of sad faces is related to activation in the amygdala, insula, thalamus, and lingual gyrus.28 Interestingly, these regions are progressively affected during the spread of 43kDa TAR DNA binding protein (TDP-43) pathology through the clinical stages of ALS.29,30 A recent study investigated metabolic correlates of processing emotional faces in patients with ALS and reported increased metabolism in the right inferior frontal gyrus and decreased activity in the hippocampi during processing sad faces.5 In sum, impairment in sadness recognition in ALS suggests pathological involvement of insula, limbic structures, and prefrontal regions during the course of the disease, but neuropathological studies are warranted to confirm this hypothesis. Deficits in other emotions such as disgust and surprise have also been reported.1
Even though it is well established that patients with ALS have impairments in emotion recognition,1 some studies in the field did not control for FTD-related behavioral symptoms.6,9,13 Interestingly, after categorizing patients with ALS into those with (ALSbs) or without (ALSns) frontal features, only the subgroup with coexisting behavioral impairment (ALSbs) was impaired in emotion recognition, as previously reported.7 Taken together, these results suggest that deficits in emotion recognition co-occur with frontal behavioral symptoms in ALS.
The study has some caveats that should be considered. The small sample size may explain some of the lack of associations found and may limit the generalizability of the results. Moreover, we did not perform neuroimaging analyses, which would be of value for understanding the neural basis of emotional recognition in ALS. Despite these limits, our results highlight the cognitive and neuropsychiatric heterogeneity of ALS and suggest that emotion recognition deficits may be a marker of frontal behavior in ALS.
1 : Meta-analysis of social cognition in amyotrophic lateral sclerosis. Cortex 2017; 88:1–7Crossref, Medline, Google Scholar
2 : Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol 2013; 12:368–380Crossref, Medline, Google Scholar
3 : The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry 2016; 87:611–619Crossref, Medline, Google Scholar
4 : Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18:153–174Crossref, Medline, Google Scholar
5 : Perception of emotional facial expressions in amyotrophic lateral sclerosis (ALS) at behavioural and brain metabolic level. PLoS One 2016; 11:e0164655Crossref, Medline, Google Scholar
6 : Emotional responding in amyotrophic lateral sclerosis. J Neurol 2005; 252:1517–1524Crossref, Medline, Google Scholar
7 : Emotion processing deficits distinguish pure amyotrophic lateral sclerosis from frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15:39–46Crossref, Medline, Google Scholar
8 : Theory of mind and its neuropsychological and quality of life correlates in the early stages of amyotrophic lateral sclerosis. Front Psychol 2016; 7:1934Crossref, Medline, Google Scholar
9 : Emotional perception deficits in amyotrophic lateral sclerosis. Cogn Behav Neurol 2007; 20:79–82Crossref, Medline, Google Scholar
10 : Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology 2011; 25:53–65Crossref, Medline, Google Scholar
11 : How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph Lateral Scler 2011; 12:45–51Crossref, Medline, Google Scholar
12 : Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology 2014; 82:149–155Crossref, Medline, Google Scholar
13 : Multimodal emotion processing deficits are present in amyotrophic lateral sclerosis. Neuropsychology 2017; 31:304–310Crossref, Medline, Google Scholar
14 : Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 2008; 119:497–503Crossref, Medline, Google Scholar
15 : Amyotrophic lateral sclerosis in Brazil: Case series and review of the Brazilian literature. Amyotroph Lateral Scler Frontotemporal Degener 2016; 17:282–288Crossref, Medline, Google Scholar
16 : [Suggestions for utilization of the Mini-Mental State Examination in Brazil]. Arq Neuropsiquiatr 2003; 61(3B):777–781Crossref, Medline, Google Scholar
17 : The effects of gender, age, schooling, and cultural background on the identification of facial emotions: a transcultural study. Int Psychogeriatr (Epub ahead of print, May 25, 2018)Google Scholar
18 : [Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD]. Rev Saude Publica 1995; 29:355–363Medline, Google Scholar
19 : The Cambridge Behavioural Inventory revised. Dement Neuropsychol 2008; 2:102–107Crossref, Medline, Google Scholar
20 : Caregiver burden in amyotrophic lateral sclerosis is more dependent on patients’ behavioral changes than physical disability: a comparative study. BMC Neurol 2012; 12:156Crossref, Medline, Google Scholar
21 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477Crossref, Medline, Google Scholar
22 : The prevalence of depressive symptoms in frontotemporal dementia: a meta-analysis. Dement Geriatr Cogn Disord 2015; 39:257–271Crossref, Medline, Google Scholar
23 : Depression and anxiety in a case series of amyotrophic lateral sclerosis: frequency and association with clinical features. Einstein (Sao Paulo) 2017; 15:58–60Crossref, Medline, Google Scholar
24 : Depression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2011; 12:109–112Crossref, Medline, Google Scholar
25 : Depression and wish to die in a multicenter cohort of ALS patients. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16:265–273Crossref, Medline, Google Scholar
26 : White matter change with apathy and impulsivity in frontotemporal lobar degeneration syndromes. Neurology 2018; 90:e1066–e1076Crossref, Medline, Google Scholar
27 : Determinants of theory of mind performance in Alzheimer’s disease: A data-mining study. Cortex 2017; 88:8–18Crossref, Medline, Google Scholar
28 : Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 2009; 34:418–432Medline, Google Scholar
29 : Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nat Rev Neurol 2013; 9:708–714Crossref, Medline, Google Scholar
30 : Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex 2018; 101:163–171Crossref, Medline, Google Scholar



