Klüver-Bucy Syndrome Following Traumatic Brain Injury: A Systematic Synthesis and Review of Pharmacological Treatment From Cases in Adolescents and Adults
Abstract
Klüver-Bucy syndrome (KBS) is a rare clinical presentation following traumatic brain injury (TBI). Symptoms include visual agnosia, placidity, hyperorality, sexual hyperactivity, changes in dietary behavior, and hypermetamorphosis. The purpose of this article was to identify and synthesize the available evidence from case reports and case series on the treatment profile of KBS among adolescents and adults after TBI. Four bibliographic databases (MEDLINE OVID, EMBASE, PsycINFO, and SCOPUS) were searched for relevant literature. No date or language restrictions were applied. All case reports containing original data on KBS following TBI among adolescents and adults were included. Articles were evaluated, and data were extracted according to predefined criteria. The literature search identified 24 case reports of KBS post-TBI published between 1968 and 2017. Most case subjects were male (70.1%), and the mean age at injury was 25.1 years (range, 13–67 years). Injury to one or both temporal lobes occurred in most cases. Inappropriate sexual hyperactivity was the most common KBS symptom, followed by a change in dietary behavior and hyperorality. Visual agnosia was the least reported. In 50% of cases, the patient fully recovered from KBS. One-half of all participants described pharmacological management; the most common medication prescribed was carbamazepine. Overall, there was a lack of data available on pharmacotherapy initiation and duration. The complex presentation of KBS presents challenges in terms of treatment options. Although overall individuals who were prescribed carbamazepine had positive outcomes, given the reliance on case reports, it is difficult to make a definitive recommendation to guide clinical practice.
Klüver-Bucy syndrome (KBS) is a rare neuropsychiatric disorder that can occur following traumatic brain injury (TBI). The syndrome was first described in 1937 as an experimental neurobehavioral syndrome in monkeys with bitemporal brain lesions1; both transient2 and permanent KBS among humans3 have been subsequently observed. The syndrome is characterized by complex behaviors, including placidity, visual agnosia, altered sexual activity, hyperorality, memory disorders, hypermetamorphosis, and emotional and nutritional behavior changes.4–6 Psychological status among those with KBS depends not only on the extent and location of the lesion but also on the level of emotional and intellectual development prior to the injury and the extent of social stimulation following brain damage. Diagnosis of KBS does not require all symptoms to be manifested simultaneously, and fully symptomatic KBS is rare.3,6,7
KBS is usually associated with lesions of the amygdala or amygdaloid pathways. It has been reported among patients displaying a variety of pathologies, including herpes simplex encephalitis,8 Huntington’s disease,9 Alzheimer’s disease,10 adrenoleukodystrophy,11 heat stroke,12 meningitis,13 multiple sclerosis,14 Pick’s disease,15 temporal epilepsy,16 and Reye's syndrome.17 These neurological disorders are associated with destruction or dysfunction of bilateral mesial temporal lobe structures.7 There are currently no definitive KBS treatment recommendations. KBS symptoms are managed on a symptom-by-symptom basis, as opposed to with pharmacotherapy prescribed for KBS as a whole.
Given the rare presentation of KBS in both clinical practice and the academic literature, our understanding of KBS following TBI has primarily relied on case reports, because of the lack of available higher quality data. The French Physical Medicine and Rehabilitation Society (SOFMER) guidelines for the care management of behavioral disorders following TBI reported level 4 grade C evidence for treatment with carbamazepine on the basis of the experience of four patients with posttraumatic lesions localized bitemporally who developed KBS.18 For the four patients in the case series, the article reported that several symptoms responded dramatically to carbamazepine.19,20 The symptoms were not specified. However, of the four patients, two had head injuries of indeterminate origin.19,20 The guidelines concluded that carbamazepine is a useful agent in the treatment of this unusual syndrome.
The aim of this study was to build on the findings reported in the SOFMER guidelines with a more comprehensive review of case reports. Therefore, the initial objective of this systematic review was to use case reports to describe KBS among adolescents and adults who had sustained a TBI. The second objective was to compare and contrast individual case reports for patients with KBS post-TBI for whom prescription of pharmacotherapies was reported. Given the potential impact of developmental issues among children, this review focuses on adolescents and adults. Although a small number of reviews have considered KBS following TBI,7,21 as far as we are aware, KBS following TBI has not been the focus of a systematic review.
This review forms part of a larger project to synthesize the evidence for the pharmacological management of neurobehavioral symptoms post-TBI as a prelude to the development of a clinical guideline. This systematic review, in addition to academic and clinical expert consensus, contributes toward the development of evidence-based recommendations for the pharmacological management of complex neurobehaviors, including KBS among patients post-TBI.
Methods
Prior to commencing the review, we searched the PROSPERO Database and Joanna Briggs database to ensure the proposed work was not duplicating any work currently in progress. This systematic review is reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines.
Inclusion and Exclusion Criteria
The present review was limited to case reports and case series of human participants available as full-text articles. Case reports and series of adolescents and adults (age: ≥13 years), both male and female, who had sustained a TBI displaying partial or full KBS symptoms were considered for inclusion. TBI was defined as an alteration in brain function or other evidence of brain pathology caused by an external force. Studies were included regardless of the severity of the injury or mechanism of injury. Participants with penetrating and nonpenetrating head injury were eligible for inclusion.
The following are considered characteristic KBS symptoms:6 placidity (loss of normal anger or fear); hyperorality (tendency to explore objects in the mouth); visual agnosia (inability to recognize objects without loss of gross visual discrimination); hypermetamorphosis (tendency to attend to and manipulate objects in the visual field); indiscriminate dietary behavior, including hyperphagia (an excessive, insatiable appetite); and hypersexuality. Diagnosis of the full form of the syndrome is based on the occurrence of all the aforementioned symptoms.6 Diagnosis of the partial form of the syndrome requires the presence of three or more symptoms.4,6 Studies in which diagnosis of KBS was based on fewer than three symptoms were excluded from the review.
To be included, case reports were required to show medical evidence of TBI—that is, unequivocal TBI documented in medical records or other health or medical reports cited by the research team associated with the published article. Examples of unequivocal evidence include findings from brain imaging (e.g., computed tomography scan [CT], magnetic resonance imaging [MRI]), Glasgow Coma Scale (GCS) score, posttraumatic amnesia, and loss of consciousness.
Studies in a language other than English were included if a translation service was available to the researchers. The following studies were excluded: case reports or series of children up to the age of 13 years; case reports or series in which the mechanism of injury was not clearly stated and it was therefore not possible to conclude that TBI was the focus; and case studies based on self-report of TBI from either the individual or an informant, in the absence of other medical evidence regarding the head injury, as outlined above.
A description of the syndrome post-TBI focusing on demographic and injury characteristics, brain imaging, symptoms, and pharmacotherapy is provided. The outcomes that were the focus of this review were treatment effectiveness, as measured by resolution or improvement in KBS symptoms; overall recovery; and harms, including adverse events resulting from prescribed pharmacotherapy.
Search Strategy
The search strategy was developed on the basis of the population-intervention-comparator-outcome elements of relevance to this review (population in this case was KBS secondary to TBI). It included a range of Medical Subject Headings terms and keywords linked by Boolean operators. The searches were carried out on December 20, 2016, and repeated on November 5, 2017. Terms were modified as appropriate for each database. The following databases were searched with no date, age, or language limitation: MEDLINE: OVID SP interface; EMBASE: Excerpta Medica Database, PsycINFO: OVID SP interface; and SCOPUS. The MEDLINE search terms and number of results for each database are included in the online supplement.
To ensure the search was as comprehensive as possible, we supplemented the formal search of bibliographic databases with searches of Google Scholar and ResearchGate. We also carried out electronic searching of the following online journals: Brain Injury, Neuropsychology, Journal of Neurotrauma, Neurocase, and BMJ Case Reports. We reviewed reference lists and bibliographies of retrieved articles to identify research not located through other search strategies. Finally, we asked colleagues whether they were aware of any case reports in this area.
Study Selection
Results from the four database searches were downloaded into Endnote×7 and deduplicated. Titles and abstracts were screened by two independent reviewers (FC, AK) against the inclusion and exclusion criteria for the review. Studies that potentially met the inclusion criteria at the title and abstract stage were retrieved in full and independently assessed against the inclusion criteria by two members of the review team (FC, AK). An article could be included in the review if it contained at least one relevant case. Articles that included both relevant cases and cases that did not meet the inclusion criteria (e.g., because of patient age or KBS not being secondary to TBI) were still eligible. Discussion between reviewers was used to achieve agreement over the eligibility of two studies. Full-text studies that did not meet the inclusion criteria were excluded, and reasons for exclusion are provided in Figure 1.
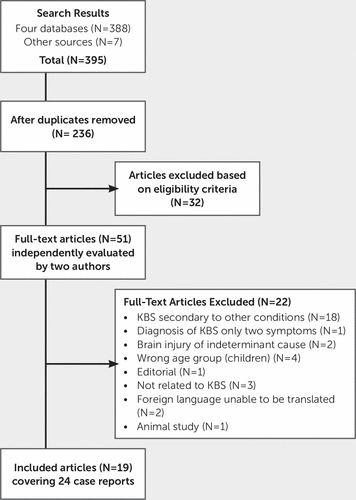
FIGURE 1. Studies Included and Excluded in the Analysis
a KBS=Klüver-Bucy syndrome.
Assessment of Methodological Quality
Quality was assessed by three reviewers (FC, AK, EA) using the Joanna Briggs Institute critical appraisal eight-question checklist for case reports. (This checklist is included in the online supplement.) In brief, the questions assess the quality of reporting of the patient’s demographic characteristics, current clinical condition, diagnostic tests or assessment methods, interventions or treatment, postintervention clinical condition, harms and unexpected consequences, and take-home lessons.
Data Extraction
Where available, the following data were extracted from the included case reports with a customized data-extraction tool pilot tested on two case reports of KBS secondary to herpes simplex virus (FC, AK). Minor modifications to the data-extraction tool were made following the pilot. To aid interpretation of data, we sought to ensure that one of the reviewers involved in data extraction had a medical degree (AK). The specific data items extracted, where available, are as follows.
We extracted data on country of the case; age, gender, and mechanism of injury of the case; and report of loss of consciousness (on the basis of GCS score and presence or absence of coma). We also gathered information on the study population: TBIs (injuries other than to the head, time postinjury), KBS syndrome symptoms, whether KBS was full or partial, time at KBS onset, and duration of KBS. We extracted data on the imaging used (CT, MRI) and on the pharmacotherapy intervention if one was used, including type of pharmacological compound, dose, frequency and duration, and cointerventions and their details. We tracked outcomes and when they were measured as well as treatment response and treatment adverse events.
Results
Study Selection
The search strategy resulted in an initial yield of 388 references from four bibliographic databases. Seven potentially relevant references were identified from Google Scholar. After removal of duplicates, 236 references remained. Of these, 185 references were excluded on the basis of either title or abstract.
The full text of 51 articles was retrieved for detailed evaluation; after independent review by two authors (FC, AK), 32 articles did not meet the inclusion criteria. Primary reasons for exclusion included incorrect study design (not a case report) or population out of scope (studies of children or animals, KBS secondary to conditions other than TBI). One study published in Italian was translated by a graduate student. One potential case report published in Polish was excluded after translation by a staff member. An English version of a further paper published in Polish was available; therefore, the Polish version was excluded. After independent review, 19 articles referring to 24 case reports met inclusion criteria for the review. The search strategy is provided in Figure 1.
Data Synthesis
The findings of the 24 cases are presented as a narrative descriptive synthesis structured around the individual case report characteristics, injury severity, brain imaging, KBS symptoms, use of pharmacotherapy, and treatment outcomes.
Demographic Characteristics
The included case reports were published between 1968 and 2017. One-half of the case reports (N=12) were from the United States, with the remainder from India (N=2),22,23 Turkey (N=1),24 Japan (N=1),25 Italy (N=1),26 Poland (N=4)19,20,27 and the Netherlands (N=3).28 Of the included case reports, 70.1% related to men and boys (N=17). The mean age at injury for the 21 case reports specifying age was 25.1 years, with a range of 13–67 years. Three further reports specified either early 20s or late 20s.7,29 The most common mechanism of injury was road traffic accident (N=17, 70.1%). There were three case reports of KBS after gunshot injury,7,35,36 and two each after a fall19,20,32 or sport-related accident.7,33 Demographic, brain imaging, and injury characteristics of case subjects in the included studies are presented in Table 1.
| Study | Case | Age at Injury (Years) | Sex | Country | Mechanism of injury | Loss of Consciousness | Glasgow Coma Scale (GCS) Scorea | Brain MRI and Computerized Tomography Results |
|---|---|---|---|---|---|---|---|---|
| Aygun et al.24 | 29 | Male | Turkey | Road traffic accident | No | 14 | Contusions of bilateral temporal lobes | |
| Bhat et al.22 | 30 | Male | India | Road traffic accident | Not reported | Not reported | Mild right anterior temporal gyrus atrophy | |
| Caro and Jimenez7 | Late 20s | Male | United States | Sport | No | Increased size of frontal lobes; right parietal white matter lesions; infratentorial lesions in cerebellar hemispheres | ||
| Deginal and Changty23 | 16 | Male | India | Road traffic accident | Yes | 10 | Bilateral temporal contusions with edema | |
| Fiume and Fiume Garelli26 | 23 | Male | Italy | Road traffic accident | Comab | Imaging not conducted; craniotomy subtemporal | ||
| Góscínski et al.19,20 | 1 | 23 | Male | Poland | Road traffic accident | Yes | 4 | Left subdural hematoma |
| Right-sided cerebral edema | ||||||||
| Góscínski et al.19,20 | 2 | 67 | Male | Poland | Fall | Yes | 7 | Intracerebral bitemporal hematoma |
| Hardy and Aldridge2 | 16 | Male | United States | Firearm | No | Right temporal lobe lesion | ||
| Hooshmand et al.34 | 16 | Female | United States | Road traffic accident | Yes, coma | Right temporal lobe damage | ||
| Isern31 | 37 | Female | United States | Firearm | No | Bitemporal lobe damage | ||
| Kwiatwoski et al.27 | 1 | 16 | Male | Poland | Road traffic accident | Yes | 3 | Bilateral contusions: temporal and parietal lobes |
| Hemorrhage into subcortical nuclei; paracerebral hematoma | ||||||||
| Kwiatwoski et al.27 | 2 | 16 | Female | Poland | Road traffic accident | Yes, GCS score=3 | Left temporal subdural hematoma | |
| Right hemisphere hemorrhagic contusion and cerebral edema | ||||||||
| Lilly et al.6 | 57 | Male | United States | Road traffic accident | Yes | Bilateral damage to inferior temporal lobes | ||
| Morcos and Guirgis32 | 39 | Male | United States | Fall | Yes | Inferior left temporal damage | ||
| Moviat et al.28 | 1 | 13 | Female | Netherlands | Road traffic accident | <7 | Left-sided parietal subdural hematoma; generalized edema | |
| Loss of basal cisterns; left temporal plus right frontotemporal contusions | ||||||||
| Moviat et al.28 | 2 | 13 | Female | Netherlands | Road traffic accident | 7 | Left-sided parietal subdural hematoma; generalized edema | |
| Loss of basal cisterns; bilateral frontotemporal contusion | ||||||||
| Moviat et al.28 | 5 | 14 | Male | Netherlands | Road traffic accident | GCS score=7 | Right frontotemporal hematoma; right frontal atrophia | |
| Hypodensity in left thalamus | ||||||||
| Salim et al.48 | 24 | Female | United States | Road traffic accident | No | 14 | Focal axonal injury to left temporal lobe | |
| Slaughter et al.29 | 1 | Late 20s | Male | United States | Road traffic accident | Yes | 3Tc | Left intraparenchymal hematoma; subarachnoid hemorrhage |
| Left temporal lobe contusions; bilateral enlargement–temporal horns | ||||||||
| Slaughter et al.29 | 2 | Early 20s | Male | United States | Road traffic accident | Yes | 6 | Bilateral small frontal lobe contusions |
| Smigielski and Boeve36 | 25 | Female | United States | Road traffic accident | Not reported | Not reported | Bilateral temporal lobe contusions; left-sided frontal injury | |
| Stewart35 | 20 | Male | United States | Road traffic accident | Not reported | Not reported | Bilateral frontal and temporal encephalomalacia | |
| Yoneoka et al.25 | 17 | M | Japan | Sport | Yes, coma | Right-sided acute subdural hematoma with transtentorial herniation | ||
| York and McCarter30 | 25 | Male | United States | Firearm | 5Tc | Bifrontal and temporal lobe contusions |
TABLE 1. Demographic and Injury Features of Klüver-Bucy Syndrome After TBI Among Case Subjects (N=24)
Injury Severity
One-half of the case subjects had sustained a severe head injury, as defined by a GCS score <8, coma, and recorded loss of consciousness. Duration of loss of consciousness was reported in only one case report. The GCS score was recorded in 13 reports, with a reported score <8 in nine cases. Coma was documented in three case reports.25,26,34 There were four cases in which loss of consciousness was noted, but the GCS score was not provided. In three cases, injury severity was unavailable because no information on the GCS score, coma, or loss of consciousness was reported.22,35,36
Brain Imaging
Brain imaging (MRI or CT) was available for most of the cases (N=23). Lesions were most commonly found in the temporal lobes, with temporal lobe lesions noted in 18 (90%) cases. Of the 15 case subjects (75%) with lesions isolated to the temporal lobes only, 11 showed bilateral lesions, and two had unilateral lesions (left, N=1; right, N=1). The frontal lobes were involved in four cases. In three of these cases, the temporal lobes were also involved.
KBS Symptoms
Sexual hyperactivity or inappropriate sexual behavior was the most common KBS symptom, noted in 95.8% of the case reports (N=23). A change in dietary habits to hyperphagia or bulimia was reported in 20 cases, with hyperphagia being the most common presentation (13 cases). Hyperorality occurred in 17 cases, and hypermetamorphosis was present in 13 cases. Placidity was reported in 15 cases. For some of these cases, the placidity occurred intermittently with agitation and aggression. Visual agnosia was the least common KBS symptom, with only eight cases reporting visual agnosia. Full and partial KBS were based on the number of presenting KBS features; in the majority of included cases, the KBS was partial (N=19; 79.1%), with the remainder being full (N=5; 20.9%).6,23–25,31
With respect to associated clinical findings, seizures were reported in four cases, and problems with memory were reported for 16 cases. Challenging behaviors associated with KBS, including impulsivity, recalcitrance, agitation, and aggression (often extreme), were noted in 16 cases.
In the majority of cases, KBS symptoms appeared early post-TBI. In 12 cases this occurred in less than 7 days post-TBI, and in five cases it occurred between 7 and 31 days post-TBI. No cases of KBS were diagnosed during coma or when a period of stupor was described.25 Symptoms of KBS appeared around 6 months post-TBI for one case,34 1 year post-TBI for two cases,22,28 and 2 years post-TBI for one case.28 For two cases it was not possible to determine the time at onset. KBS features, including time at onset, and associated clinical findings for each of the included case reports are presented in Table 2.
| Study | Case | Time at KBS Onsetb | Memory | Visual Agnosia | Placidity | Change in Diet | Hyperoral | Sexual Hyperactivity | Hypermetamorphoses | Behavior | Full or Partial KBS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aygunet al.24 | T1 | Not described | + | + | + | + | + | Not described | FA | Full | |
| Bhat et al.22 | T3 | Not described | + | + | Not described | + | + | + | FA | Partial | |
| Caro and Jimenez7 | T5 | Not described | Not described | + | + | + | + | Not described | A | Partial | |
| Deginal and Changty23 | T2 | Not described | + | + | + | + | + | + | A | Full | |
| Fiume and Fiume Garelli26 | T1 | + | Not described | + | + | + | + | + | D | Partial | |
| Góscínski et al.19,20 | 1 | T2 | + | Not described | Not described | + | + | + | Not described | A/FA | Partial |
| Góscínski et al.19,20 | 2 | T2 | Not described | Not described | Not described | + | + | + | + | A | Partial |
| Hardy and Aldridge2 | T1 | + | + | + | + | Not described | + | + | FA | Partial | |
| Hooshmand et al.34 | T3 | + | Not described | Not described | + | + | + | Not described | A | Partial | |
| Isern31 | T1 | + | + | + | + | + | + | + | A | Full | |
| Kwiatwoski et al.27 | 1 | T1 | Not described | + | + | + | + | + | Not described | A | Partial |
| Kwiatwoski et al.27 | 2 | T1 | + | Not described | Not described | + | + | + | + | A/D | Partial |
| Lilly et al.6 | T2 | + | + | + | + | + | + | + | FA/R | Full | |
| Morcos and Guirgis32 | T1 | + | Not described | Not described | + | Not described | + | + | A | Partial | |
| Moviat et al.28 | 1 | T4 | + | Not described | + | + | Not described | + | + | RE | Partial |
| Moviat et al.28 | 2 | T3 | + | Not described | + | Not described | Not described | + | + | RE | Partial |
| Moviat et al.28 | 5 | T1 | + | Not described | + | + | + | + | Not described | RE | Partial |
| Salim et al.48 | T1 | + | Not described | + | + | Not described | + | Not described | A | Partial | |
| Slaughter et al.29 | 1 | T2 | + | Not described | Not described | + | + | + | Not described | A/R | Partial |
| Slaughter et al.29 | 2 | T1 | + | Not described | Not described | Not described | + | N | Not described | A/FA/R | Partial |
| Smigelski and Boeve36 | T5 | Not described | Not described | Not described | Not described | + | + | Not described | A | Partial | |
| Stewart35 | T1 | + | Not described | + | + | Not described | + | + | A | Partial | |
| Yoneoka25 | T2 | Not described | + | + | + | + | + | + | N/R | Full | |
| York and McCarter30 | T1 | + | Not described | Not described | + | Not described | + | Not described | N/R | Partial |
TABLE 2. Klüver-Bucy Syndrome (KBS) Symptoms and Featuresa
Recovery Over Time: All Case Subjects (N=24)
Response to treatment and improvement in KBS features are summarized in Table 3. Of the case reports included in this review, mention of recovery from KBS was noted in 22 cases (a report on recovery was not available for two case subjects). For the 11 case subjects who showed positive improvement in all presenting KBS symptoms, the duration of KBS ranged between 7 days and 14 months. Among case subjects for whom there was partial symptom improvement (N=6), some KBS symptoms fully resolved, whereas others persisted over the long-term. For five case subjects, there was no improvement, with reports of deterioration in health and reduced capacity in functioning.19,26,28 In a single case, progressive deterioration of symptoms leading to death was reported.26 There was underreporting of when KBS patients were assessed over the timeline of their hospital stay, during rehabilitation, and following hospital discharge. Assessments of long-term outcomes of 12 months or longer post-TBI were recorded in few cases (N=5).19,20,25,28
| Study | Case | KBS Symptom Improvement | Duration of KBS | Treatment Response and Outcome |
|---|---|---|---|---|
| Aygun et al.24 | Positive | 5 days | Complete improvement | |
| Bhat et al.22 | Positive | 3 weeks | Asymptomatic | |
| Caro and Jimenez7 | Partial | Gradual improvement in behavior and cognition | ||
| Deginal et al.23 | Partial | 30% Improvement in behavior and KBS symptoms when measured at 8 weeks post-TBI | ||
| Fiume and Fiume Garelli26 | No improvement | Died | Progressive deterioration leading to death | |
| Góscínski et al.19,20 | 1 | No improvement | Ongoing | Reduced intellectual capacity and functioning (7 years) |
| Irritable, verbally aggressive, poor memory, apathy, and no libido (25 years) | ||||
| Góscínski et al.19,20 | 2 | No improvement | Ongoing | Prolonged psychiatric disorders, social maladaptation, aggression, and self-harming |
| Hardy and Aldridge2 | Partial | 15 days | Improved appetite, language, and no sexual comments 15 days post-TBI | |
| Obsessive-compulsive disorder and blunt affect persisted | ||||
| Hooshmand et al.34 | Positive | 1 year | Seizures ended in 24 hours; hyperorality disappeared; concentration improved; substantial improvement in memory | |
| Isern31 | Not reported | 4–5 months | ||
| Kwiatwoski et al.27 | 1 | Positive | Managed to effectively return to preaccident functioning, including work | |
| Kwiatwoski et al.27 | 2 | Partial | Persisted symptoms of hyperorality 4 years post-TBI | |
| Improvement in dietary habits, emotional dullness, emotionality, physical violence, and anger management | ||||
| Lilly et al.6 | Positive | 1 month | ||
| Morcos and Guirgis32 | Not reported | |||
| Moviat et al.28 | 1 | No improvement | No change after 6 years rehabilitation | |
| Moviat et al.28 | 2 | No improvement | No change after 3 years rehabilitation | |
| Moviat et al.28 | 5 | Partial | 2 years | Bilateral pyramidal signs improved |
| No change after 3 years rehabilitation | ||||
| Salim et al.48 | Positive | Approximately 7 days | Resolved | |
| Slaughter29 | 1 | Positive | 3.5 months | Complete resolution |
| Slaughter et al.29 | 2 | Positive | 18 days | Reduced symptoms of agitation/lip chewing; symptoms did not recur |
| Smigelski and Boeve36 | Positive | 8 months | Impressive neurobehavioral and neurocognitive recovery | |
| Stewart35 | Positive | <3 months | Positive improvement (gradual decrease, ceased violent attacks) | |
| Yoneoka et al.25 | Positive | 14 months | Transient symptoms of KBS; returned to high school and then college | |
| York and McCarter30 | Partial | Improvement in residual memory and cognition |
TABLE 3. Response to Treatment Among Klüver-Bucy Syndrome (KBS) Case Subjects (N=12) After TBI
Pharmacological Treatment
Pharmacological management was documented in 50% of the case reports (N=12). Pharmacological management with carbamazepine was the most commonly reported intervention (N=10). The use of first-generation antipsychotics was noted in three case reports. Selective serotonin reuptake inhibitors (SSRIs) were prescribed in three cases. The prescription of two or more medications was noted in four case reports.7,27,29 Details of the postacute pharmacological management are summarized in Table 4. Only two case reports reported duration of treatment at 4 years, with the remaining case reports not reporting duration of medication treatment.
| Study | Case | Pharmacotherapy |
|---|---|---|
| Bhat et al.22 | Carbamazepine; no further details provided | |
| Caro and Jimenez7 | Valproate changed to topiramate, quetiapine, propranolol, benztropine, and haloperidol | |
| Deginal and Changty23 | Carbamazepine (200 mg b.i.d.) | |
| Góscínski et al.19,20 | 1 | Carbamazepine |
| Góscínski et al.19,20 | 2 | Carbamazepine (400 mg b.i.d.) |
| Hooshmand et al.34 | Chlorpromazine (300 mg/day) (discontinued) | |
| Carbamazepine (1,000 mg/day) (commenced after chlorpromazine) | ||
| Kwiatwoski et al.27 | 1 | Haloperidol |
| Carbamazepine administered orally three times per day (200 mg/per day); serum level 6 μg/ml | ||
| Kwiatwoski et al.27 | 2 | Carbamazepine administered orally three times per day (200 mg/day); 6 μg/ml serum level |
| Morcos and Guirgis32 | Carbamazepine (400 mg b.i.d.) | |
| Slaughter et al.29 | 1 | Carbamazepine (600 mg/day); propranolol (no change in baseline behavior) |
| Trazodone | ||
| Sertraline (titrated to 150 mg); substituted with fluoxetine (40 mg in the morning and 20 mg at noon) | ||
| Slaughter et al.29 | 2 | Haloperidol; substituted with olanzapine |
| Lorazepam, valproic acid, thiothixene, and bromocriptine | ||
| Sertraline (150 mg/per day) | ||
| Stewart 35 | Carbamazepine; serum level 9–11 μg/ml for 3 weeks, then 8–9 ug/ml for 1 year |
TABLE 4. Pharmacotherapy After TBI Among Klüver-Bucy Syndrome Case Subjects (N=12) Following Initial Treatment in the Acute Setting
Treatment With Carbamazepine
The age range of patients who received either carbamazepine or another anticonvulsive medication was 16–39 years old. In 10 of the 12 cases in which pharmacological treatment was described, carbamazepine was used to manage patients presenting with either full or partial symptoms of KBS. In these cases, the dose of carbamazepine ranged from 400 mg to 1,000 mg per day.19,20,27,29,32,34,35 Therapeutic drug monitoring to tailor carbamazepine treatment was only described in three cases and aimed to reach serum levels between 6 and 11 μg/ml. In most cases, carbamazepine was used as monotherapy, whereas in two cases carbamazepine was combined with propranolol and haloperidol, respectively, which resulted in resolution of all or the majority of symptoms.7,27,29 Unfortunately, few of the case reports described any data on the adverse effects of any drugs that were used in symptom management.
Response to Pharmacological Treatment (12 Cases)
One-half of the case reports (N=12) included in this review described pharmacological management. Of these, six cases were associated with a positive improvement in symptoms, with some reporting complete resolution of symptoms and return to preinjury activities. Partial improvement was reported in three cases, with some KBS symptoms improving over time and others persisting. In the two definitive TBI cases reported by Góscínski et al.19,20 that had a long follow-up of 3 and 25 years, respectively, prolonged psychiatric symptoms persisted with reduced capacity for functioning. In 50% of the cases that reported administration of carbamazepine, a positive improvement in KBS symptoms was noted. In two cases, a combination of drugs was used that did not include carbamazepine but did include sertraline, thiothixene, bromocriptine, topiramate, haloperidol, propranolol, and quetiapine.7,29 Both case reports noted a gradual improvement in symptoms specifically relating to agitation.
Methodological Quality
The methodological quality of each case report was examined according to the Joanna Briggs Institute critical appraisal for case reports template. The questions that form the basis of the critical appraisal are included in the online supplement. Overall there was clear reporting of the patient’s demographic characteristics (question 1), current clinical condition (question 3), and diagnostic tests or assessment methods (question 4). Only 13 case reports (54.1%) clearly described the patient’s history in a timeline by providing information on past medical history and relevant family history (question 2), and only seven case reports provided information on harms and unexpected consequences (question 7). Critical appraisal findings for the included KBS cases are presented in Table 5.
| Study | Case | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 |
|---|---|---|---|---|---|---|---|---|---|
| Aygun et al.24 | Y | Y | Y | Y | Y | N/A | N | Y | |
| Bhat et al.t22 | Y | Y | Y | Y | Y | Y | N | Y | |
| Caro and Jimenez7 | Y | Y | Y | Y | Y | Y | Y | Y | |
| Deginal and Changty23 | Y | Y | Y | Y | Y | Y | N | Y | |
| Fiume and Fiume Garelli26 | Y | N | Y | Y | N | N | N | N | |
| Góscínski et al.19,20 | 1 | Y | N | Y | Y | N | N | N | Y |
| Góscínski et al.19,20 | 2 | Y | N | Y | Y | N | N | N | Y |
| Hardy and Aldridge2 | Y | N | Y | Y | N | Y | N | N | |
| Hooshmand et al.34 | Y | Y | Y | Y | Y | Y | Y | Y | |
| Isern31 | Y | Y | Y | Y | Y | Unclear | N | N | |
| Kwiatwoski et al.27 | 1 | Y | Y | Y | Y | Y | Y | N | Y |
| Kwiatwoski et al.27 | 2 | Y | Y | Y | Y | Y | Y | N | Y |
| Lilly et al.6 | Y | N | Y | Y | Y | Y | N | Y | |
| Morcos and Guirgis32 | Y | Y | Y | Y | Y | Y | N | Y | |
| Moviat et al.28 | 1 | Y | N | Y | Y | N | N | Y | Y |
| Moviat et al.28 | 2 | Y | N | Y | Y | N | N | Y | Y |
| Moviat et al.28 | 5 | Y | N | Y | Y | N | N | Y | Y |
| Salim et al.48 | Y | N | Y | Y | N/A | N | N | Y | |
| Slaughter et al.29 | 1 | Y | Y | Y | Y | Y | Y | Y | Y |
| Slaughter et al.29 | 2 | Y | N | Y | Y | Y | Y | Y | Y |
| Smigelski and Boeve36 | Y | N | Y | Y | N | Y | N | N | |
| Stewart35 | Y | Y | Y | Y | Y | Y | N | Y | |
| Yoneoka et al.25 | Y | Y | Y | Y | Y | Y | N | Y | |
| York and McCarter30 | Y | Y | Y | Y | N | N | N | Y |
TABLE 5. Critical Appraisal of Post-TBI Klüver-Bucy Syndrome (KBS) Case Reports According to the Joanna Briggs Institute Appraisal Checklist for Case Seriesa
Discussion
To the best of our knowledge, this is the first systematic review to identify and synthesize the evidence for KBS secondary to TBI from case reports. Using a defined search strategy applied to four bibliographic library databases, we identified 24 case reports that met the inclusion criteria and form the focus of this review. Notwithstanding the methodological limitations associated with case reports, given the rarity of KBS and the lack of studies with higher quality methodologies, the use of case reports offers opportunities to better understand KBS post-TBI.
In the current review, hypersexuality, hyperorality, and aggressive behavior were the most common presenting KBS symptoms. Visual agnosia was the least common symptom. Gerstenbrand et al.37 summarized their experiences with posttraumatic cases of KBS, reporting clinical data on 40 case subjects diagnosed between 1978 and 1981. Although no individual case data were presented, the frequency of KBS symptoms was reported. Symptoms of bulimia, memory disturbances, hyperorality, and visual agnosia occurred in 30 cases. Hypersexuality occurred in 18 cases, and aggressiveness occurred in 11 cases. Persistence of hypersexuality at 1 year was noted in 12 cases, bulimia in eight cases, and aggressiveness in 10 cases. Although the sources of the cases are not described, the majority were male (77.5%), and the age range indicates the cohort was younger, because children were included (aged 7–33 years [mean=17.2 years]). The differences may reflect the heterogeneous nature of TBI. It is unclear whether the patients in the study by Gerstenbrand et al.37 had TBI or other acquired brain injury. Six case subjects died within 10 weeks of their accident, and thus it is possible that the cases described in the current review were less serious.
Although the natural history of KBS post-TBI is not known,23 it appears as a chronic, persistent state for some patients and a transient, resolving state for others.6 Of the case reports described in the current review, the majority of cases showed partial or full recovery. For the majority of cases, the course of KBS ranged from 5 days to 1 year. For other cases (N=8), the syndrome was ongoing 1–25 years following onset, depending on when the patient was evaluated. Although it cannot be ruled out that this reflects a positive-outcome publication bias, the extent of recovery is consistent with the study by Formisano et al.,38 who examined the global outcome of 19 patients with KBS secondary to severe brain injury following a traffic accident. To be included in Formisano et al.’s study, cases were counted as KBS if they had two of the KBS symptoms.38 This is at odds with the definition of partial KBS, which requires three symptoms.6 Although no individual case data were presented, of the 19 included KBS patients, four did not regain independence, six achieved family integration, and nine achieved work integration. In keeping with Formisano et al.’s38 findings, lesions in the temporal lobes were common in the case reports included in the current synthesis.
The nature and anatomic location of the lesions necessary to produce human KBS post-TBI have not been definitively determined, in part because of the limits of current routine structural imaging. Investigators have proposed a number of hypotheses. Góscínski et al.19,20 proposed a bilateral injury of the mediobasal temporal lobe as a result of swelling or edema of the brain and compression of the arteries. Yoneoka et al.25 hypothesized that KBS symptomatology may reflect edema-induced transient dysfunction of the right temporal and basal frontal lobes. Slaughter et al.29 proposed combinations of posterior frontal and anterior temporal lobe defects, and Deginal and Changty23 postulated disruptions of pathways connecting the dorsomedial thalmi with prefrontal cortices and other limbic areas. It is notable that the extent of neurological deficits did not correlate with the level of personality disturbances.27 More recently, Caro and Jimenez7 proposed that KBS results from mesiotemporal lesions or other changes (possibly transient) leading to hypofunctioning in the amygdala or its projections, regardless of etiology. In the case of transient KBS, they proposed that the disappearance of KBS symptoms follows improvement in the localized neuronal dysfunction.
A secondary objective of the current review was to compare and contrast the individual case reports that described pharmacological management of KBS-related symptoms. The French SOFMER guidelines on drugs for behavioral disorders following TBI reported level 4 grade C evidence for the use of carbamazepine.18 This was based on a single case series of four patients treated with carbamazepine.19,20 No data on duration of treatment or time point at which treatment was initiated were provided. Dose of carbamazepine was not reported for case 1.
The authors reported that the patients showed improvement, as measured by the Glasgow Outcome Scale (GOS), at hospital discharge, 3 months, and 6 months after trauma when given carbamazepine during hospitalization and after discharge.19,20 Case 2 did not show a change in GOS score. The nature of the improvement was not described. Given the complexity of the syndrome, the fact that only two cases can be definitively considered as TBI, the lack of pharmacological information, and the lack of reporting on which symptoms improved, guideline recommendations based on this article should at best be circumspect.
Ten case reports that described treatment with carbamazepine were included in this review, and they provide some additional evidence on the use of carbamazepine in relation to KBS. Improvement of symptoms was seen within 3 weeks of commencing carbamazepine,22 and in one case, complete resolution of symptoms was reported,34 but no time frame was specified. Other cases reported general improvements in symptoms for patients taking carbamazepine, but, again, no time frame was mentioned when improvements occurred.23,27,34,35
Carbamazepine is a carboxamide-derivate antiepileptic drug with a chemical structure similar to tricyclic antidepressants and is used in the management of seizure disorders, neuropathic pain, and psychiatric disorders.39 The efficacy of carbamazepine as an anticonvulsant drug has been demonstrated among patients with temporal lobe epilepsy40 with additional symptoms similar in nature to KBS, which could be one of the reasons behind its use. Moreover, both full and partial remission of symptoms following carbamazepine treatment have been noted in KBS associated with a range of etiologies other than TBI. Unfortunately, the case reports in the current study did not report in detail which of the diagnostic KBS symptoms improved. Improvements in behavioral, hyperorality, and cognitive symptoms were documented.
Of the 24 cases described in this systematic review, 10 were treated with carbamazepine. Overall, the cases suggest that carbamazepine may be an effective treatment for certain symptoms of KBS. Of the cases treated with carbamazepine, a serum carbamazepine trough level of >6 µL/mL was reported for three patients (optimal therapeutic range: 4–12 µL/mL). Because of its potentially severe toxic effects (e.g., ataxia, seizures, dystonic reaction, and even coma), it is crucial to avoid carbamazepine overdosing.39 Although a serum trough level of 6 µL/ml is still within the recommended range, the lack of effect with carbamazepine in Slaughter et al.’s29 case 1 maybe associated with a subtherapeutic trough level.
The antipsychotic drug chlorpromazine usually shows a high sedative effect and increased potential for extrapyramidal symptoms. In the case presented by Hooshmand,41 chlorpromazine might have been given as a treatment for psychotic symptoms, and the treatment then could have been switched to carbamazepine for long-term seizure management.34 In terms of effectiveness, the early introduction of carbamazepine seems to be important, as stated by Góscínski et al.19,20
The prescription of multiple medications was described in four cases. In Slaughter et al.’s29 case 2, both lorazepam and valproic acid were prescribed. Lorazepam is usually prescribed for its anxiolytic effects, and its reported pharmacokinetic interaction with valproic acid might lead to further aggravated sedative effects.42 In Slaughter et al.’s29 case 1, the patient received several antidepressants, including sertraline and trazodone. Sertraline was later substituted with fluoxetine; both drugs are SSRIs. Some KBS symptoms have obsessive-compulsive disorder (OCD) features, and sertraline at higher doses has been shown to be effective with OCD.43 However, sertraline and fluoxetine are both metabolized on common cytochrome P450 pathways, including CYP2D6, and pharmacokinetic interactions might be expected in the present case given the rapid succession.44 The antidepressant trazodone, a potent serotonin and α1-adrenergic receptor antagonist and weak serotonin reuptake inhibitor, was administered together with carbamazepine.45 With carbamazepine being a known inducer of CYP3A4, potential interactions might be clinically relevant and exacerbate symptom remission.46,47
It is important to acknowledge that this systematic review is subject to limitations that are a feature of case reports. Because case reports are retrospective observational studies, causal inference cannot be made. There is a risk of positive-outcome bias, and one might overinterpret features of the case because of the different emphasis given by authors to cases. For the current review, data were missing for some of the collected attributes. Underreporting of when KBS patients were assessed during their hospital stay, during rehabilitation, and following hospital discharge limits the interpretation of the duration of KBS symptoms.
Because it is a natural experiment, the case cannot be repeated, and the care of patients with KBS might have changed over the years. Because peer-reviewed articles on KBS are not very common, the search strategy did not include a large range of terms, and it is possible that relevant cases were missed. We think the likelihood of this is small given the range of databases searched, the number of reference lists consulted, and the search of Google Scholar.
Notwithstanding these limitations, the strengths of the review include the systematic identification of cases through a specified search strategy and a critical appraisal of the reports’ quality. Moreover, no date or language restrictions were applied, and we were able to get three studies translated. The review brings together 24 cases of KBS post-TBI, of which half were treated with pharmacotherapy. This review will help clinicians to understand the clinical spectrum of KBS post-TBI and may offer guidance in the personalization of treatments in clinical practice.
Conclusions
This systematic review has identified and synthesized the evidence from 24 case reports of KBS post-TBI. The case reports demonstrate the complex presentation of KBS symptoms post-TBI. Treatment with carbamazepine was associated with improvement in KBS symptoms in seven out of 10 cases. However, given the quality of evidence, it is not possible to make a practice recommendation in the clinical guideline under development with any degree of certainty. Whether this intervention is trialed for a patient relies on the individual clinical presentation, lack of response to other treatments, and clinical indication.
1 : Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry 1939; 42:979–1000Crossref, Google Scholar
2 : Traumatic transient Klüver-Bucy syndrome. Surg Neurol 1981; 15:338–340Crossref, Medline, Google Scholar
3 : The Klüver-Bucy syndrome. Front Neurol Neurosci 2018; 41:77–89Crossref, Medline, Google Scholar
4 : Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci 1997; 9:429–438Link, Google Scholar
5 : The Klüver-Bucy syndrome in man. J Nerv Ment Dis 1978; 166:130–134Crossref, Medline, Google Scholar
6 : The human Klüver-Bucy syndrome. Neurology 1983; 33:1141–1145Crossref, Medline, Google Scholar
7 : Mesiotemporal disconnection and hypoactivity in Kluver-Bucy syndrome: Case series and literature review. J Clin Psychiatry 2016; 77:e982–e988Crossref, Medline, Google Scholar
8 : Kluver-Bucy syndrome—a rare complication of herpes simplex encephalitis. J Indian Med Assoc 2006; 104:637–638Medline, Google Scholar
9 : Kluver-Bucy syndrome in Huntington’s chorea. J Nerv Ment Dis 1985; 173:632–635Crossref, Medline, Google Scholar
10 : Alzheimer abnormalities of the amygdala with Klüver-Bucy syndrome symptoms: an amygdaloid variant of Alzheimer disease. Arch Neurol 2009; 66:125–129Crossref, Medline, Google Scholar
11 : Klüver-Bucy syndrome caused by adreno-leukodystrophy. Neurology 1980; 30:1231–1232Crossref, Medline, Google Scholar
12 : Kluver-Bucy syndrome following heat stroke in a 12-year-old girl. Pediatr Neurol 1995; 13:73–76Crossref, Medline, Google Scholar
13 : Partial Kluver-Bucy syndrome secondary to tubercular meningitis. BMJ Case Rep 2016; 2016:16Google Scholar
14 Kluver-Bucy syndrome in a multiple sclerosis patient. J Neurological Sciences. 2013;1:e377–e378Crossref, Google Scholar
15 : Klüver-Bucy syndrome in Pick’s disease. Neurology 1983; 33:957–959Crossref, Medline, Google Scholar
16 : Transient Kluver-Bucy syndrome following complex partial status epilepticus. Epilepsy Behav 2003; 4:348–351Crossref, Medline, Google Scholar
17 : Single-photon emission CT and MR findings in Klüver-Bucy syndrome after Reye syndrome. AJNR Am J Neuroradiol 1997; 18:540–542Medline, Google Scholar
18 ; Drugs for behavior disorders after traumatic brain injury: sSystematic review and expert consensus leading to French recommendations for good practice. Ann Phys Rehabil Med 2016; 59:42–57Crossref, Medline, Google Scholar
19 : The Kluver-Bucy syndrome. J Neurosurg Sci 1997; 41:269–272Medline, Google Scholar
20 : The Kluver-Bucy syndrome. Acta Neurochir (Wien) 1997; 139:303–306Crossref, Medline, Google Scholar
21 : The neuropsychology of the Klüver-Bucy syndrome in children. Handb Clin Neurol 2013; 112:1285–1288Crossref, Medline, Google Scholar
22 : Partial Kluver-Bucy syndrome as a delayed manifestation of head injury. Ind Psychiatry J 2009; 18:117–118Crossref, Medline, Google Scholar
23 : Post traumatic Kluver-Bucy syndrome: a case report. Ind J Neurotrauma. 2011; 8:41–42Crossref, Google Scholar
24 : Postcontusional Kluver-Bucy syndrome. Am J Emerg Med 2003; 21:246–247Crossref, Medline, Google Scholar
25 : Human Kluver-Bucy syndrome following acute subdural haematoma. Acta Neurochir (Wien) 2004; 146:1267–1270Crossref, Medline, Google Scholar
26 : A case of Kluver Bucy syndrome probably caused by traumatic bitemporal injury. G Psichiatr Neuropatol 1968; 96:122–130Google Scholar
27 : Neuropsychological characteristic of post-traumatic Kluver-Bucy syndrome. Arch Psychiatry Psychother 2011; 13:59–65Google Scholar
28 : Kluver-Bucy syndrome in brain injured children; neurological, neuroradiological, behavioural and neuropsychological findings. J Rehabil Sciences. 1995; 8:106–109Google Scholar
29 : Selective serotonin reuptake inhibitor treatment of post-traumatic Klüver-Bucy syndrome. Brain Inj 1999; 13:59–62Crossref, Medline, Google Scholar
30 : Kluver-Bucy syndrome after facial gunshot wound: a case report. PM R 2017; 9(Supplement 1):S231Crossref, Medline, Google Scholar
31 : Family violence and the Klüver-Bucy syndrome. South Med J 1987; 80:373–377Crossref, Medline, Google Scholar
32 : A case of acute-onset partial Kluver-Bucy syndrome in a patient with a history of traumatic brain injury. J Neuropsychiatry Clin Neurosci 2014; 26:E10–E11Link, Google Scholar
33 Beyond Kluver-Bucy syndrome: colorful clinical picture of bilateral anterior cerebral artery infarction. J Neurological Sciences 2013; 1:e648Crossref, Google Scholar
34 : Klüver-Bucy syndrome. Successful treatment with carbamazepine. JAMA 1974; 229:1782Crossref, Medline, Google Scholar
35 : Carbamazepine treatment of a patient with Klüver-Bucy syndrome. J Clin Psychiatry 1985; 46:496–497Medline, Google Scholar
36 : Kluver-Bucy syndrome in traumatic brain injury: a case study. Arch Clin Neuropsychol 1997; 4:406Crossref, Google Scholar
37 : Klüver-Bucy syndrome in man: experiences with posttraumatic cases. Neurosci Biobehav Rev 1983; 7:413–417Crossref, Medline, Google Scholar
38 : Presence of Klùver-Bucy syndrome as a positive prognostic feature for the remission of traumatic prolonged disturbances of consciousness. Acta Neurol Scand 1995; 91:54–57Crossref, Medline, Google Scholar
39 : Antiepileptic drugs--best practice guidelines for therapeutic drug monitoring: a position paper by the Subcommission on Therapeutic Drug Monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008; 49:1239–1276Crossref, Medline, Google Scholar
40 . Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. P T 2010; 35(7):392-415Medline, Google Scholar
41 : Adverse effects of antipsychotic medications. Am Fam Physician 2010; 81:617–622Medline, Google Scholar
42 : Lorazepam-valproate interaction: studies in normal subjects and isolated perfused rat liver. Epilepsia 1994; 35:221–225Crossref, Medline, Google Scholar
43 : High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. J Clin Psychiatry 2006; 67:15–22Crossref, Medline, Google Scholar
44 : Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther 2008; 30:1206–1227Crossref, Medline, Google Scholar
45 : Mechanism of action of trazodone: a multifunctional drug. CNS Spectr 2009; 14:536–546Crossref, Medline, Google Scholar
46 : Probable interaction between trazodone and carbamazepine. Pharmacopsychiatry 2011; 44:158–159Crossref, Medline, Google Scholar
47 : A useful tool for drug interaction evaluation: the University of Washington Metabolism and Transport Drug Interaction Database. Hum Genomics 2010; 5:61–72Crossref, Medline, Google Scholar
48 : Klüver-Bucy syndrome as a result of minor head trauma. South Med J 2002; 95:929–931Crossref, Medline, Google Scholar



