Repetitive Transcranial Magnetic Stimulation Improves Executive Function and Music Rhythm
Although temporoparietal lobe dysfunction in the form of impaired retrieval of learned information is the cardinal symptom of Alzheimer’s dementia, frontal lobe dysfunction causes much caregiver distress and is often the presenting symptom among highly educated individuals (1). Perhaps the high educational achievement of this patient population masks the “typical” Alzheimer’s presentation, adding to clinical confusion. The frontal lobe dysfunction is often manifested as decline in motivation (apathy), cognitive rigidity and inability to perform sequential tasks (executive dysfunction), and poor insight. These atypical presentations are difficult to diagnose and manage. Many patients retain semantic memory, but their symptoms do not respond to conventional Alzheimer’s treatments, often resulting in significant caregiver burden. We present a case of a patient with a known history of Alzheimer’s dementia who presented with new-onset music rhythm perception impairment. On testing, he was found to have prominent executive dysfunction, apathy, and poor insight. Because most of his presenting symptoms were frontal lobe in origin, an off-label trial of repetitive transcranial magnetic stimulation (rTMS) to the left dorsolateral prefrontal cortex (DLPFC) using the refractory depression protocol was conducted. We examine whether rTMS improved music rhythm in this patient, and we present results after 12 consecutive rTMS sessions.
Case Report
A 72-year-old Caucasian male with a known history of Alzheimer’s disease was brought into the memory clinic with worsening of his condition. The patient was a retired hospital executive from a local community hospital with 18 years of education. At the time of his visit, he was a choir member and played the trumpet in a community band. While in college, he played the trumpet on a music scholarship but stopped playing for 40 years until he relearned how to play the instrument by self-teaching. His wife reported that he had been playing in the community band for the past 9 years and singing in the church choir regularly. About a year before the patient’s visit to the memory clinic, the music director of both the band and the choir noticed that he was late for rehearsals, very disorganized, and often lost his sheet music. The music director reported that the patient had difficulty reading the music, his pitch was off-key, and he missed the beat while playing. The director conveyed that the patient had lack of perception and judgment about his pitch and tempo being off-key and that he had no insight that he was at least a measure off and often blamed the other band members for not being in sync with him. Additionally, his wife reported that he had severe lack of initiative, motivation, and persistence, along with occasional increase in irritability. His wife also reported that he slept all day and moved slowly. The most concerning issue for her was that they had not attended church service together for nearly 4 years because he could not get himself ready in time. The patient self-reported that nothing was wrong with him and that he came to see the doctor only to please his wife.
On examination, his vitals were stable, and his physical examination was unremarkable. He was assessed for cognition, executive function, and motivation. He scored 29/30 and 98/100 on the Modified Mini-Mental State Examination (2), took 170 seconds to complete the Trail-Making Test, Part B, and scored 61/72 on the Apathy Evaluation Scale-Clinician version, indicating that he had severe apathy. The Modified Mini-Mental State Examination is a global screen for cognition and includes subtests for a broad range of cognitive capacity and difficulty levels. Scores range from 30 to 100, with higher scores indicating better cognition. The Trail-Making Test, Part B (3), is widely used for assessment of executive function, and lower scores indicate better executive function. The Apathy Evaluation Scale-Clinician version (4) measures behavioral, cognitive, and emotional domains of apathy for the previous 4 weeks. Scores range from 18 to 72, with higher scores reflecting more severe apathy. A score ≥40 is considered clinically significant apathy. The patient’s scores on the Neuropsychiatric Inventory (5), a broad screen for behavioral problems, were positive for agitation, anxiety, apathy, irritability, and disinhibition. Conners’ Continuous Performance Test (6), which measures attention, impulsivity, sustained attention, and vigilance, was also carried out. Lower values of commission error percent and hit reaction time indicate better attention and executive function. The patient’s percent commission error was 15, and his hit reaction time was 472.95. He was diagnosed with apathy and executive dysfunction.
He was offered several treatment options, including an off-label trial of repetitive rTMS and off-label use of stimulants or stimulating antidepressants, such as bupropion. Both the patient and his wife chose rTMS because it was “not adding one more medication.”
The patient received high-frequency rTMS according to the refractory depression protocol. On the first day, single-pulse TMS was used to find the scalp position of lowest motor threshold for the right first-dorsal interosseous or abductor pollicis brevis muscle with a preprogrammed algorithm for the NeuroStar device (Neuronetics, Malvern, Pa.). The left DLPFC, defined as a location 5.5 cm anterior to the motor threshold location, was targeted for stimulation. Each treatment session consisted of a total of 3,000 pulses at 10 Hz, a 4-second train duration, and a 26-second intertrain interval at 120% motor threshold. The patient received 12 daily treatments before his planned vacation to California with his wife. He tolerated the treatment without any adverse events.
Significant improvement was reported in his motivation and music performance by both his wife and music director. The patient’s wife reported that it was easier to wake him up in the morning, he napped less during the day, he helped with chores, and he finally realized that he was having some problems. His band director reported that he had a positive attitude and was “upbeat.” He started arriving to practice on time, stayed on-key, and significantly improved on his music rhythm. The director also reported that he stayed longer to practice his music and volunteered to help with the setup for the band. All outcome measures were repeated after completion of 12 rTMS sessions. The patient scored 29/30 and 99/100 on the Modified Mini-Mental State Examination, took only 126 seconds to complete the Trail-Making Test, Part B, and scored 47/72 on the Apathy Evaluation Scale-Clinician version, indicating that he had significant improvement in executive function and apathy (Figure 1). His performance on the Conners’ Continuous Performance Test also improved significantly, with commission errors (%) of 10 and a hit reaction time of 445.19.
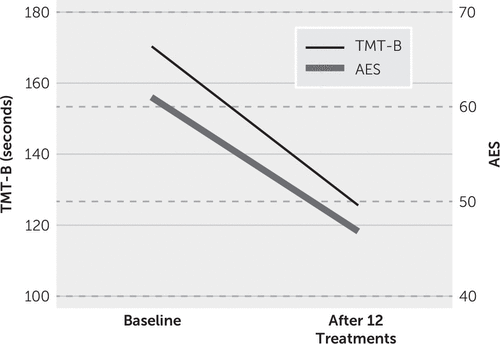
FIGURE 1. Executive function and apathy before and after transcranial magnetic stimulation in a patient with new-onset music rhythm perception impairmenta
a TMT-B=Trail-Making Test, Part B, AES=Apathy Evaluation Scale-Clinician version.
Discussion
Cognitive underpinnings of music are complex. Important aspects of music such as pitch perception, pitch memory, and rhythm perception may all be controlled by distinct, but overlapping, brain areas. The parietal, temporal, and frontal areas (left supramarginal gyrus) are critical for pitch perception and memory (7). The temporal pattern generators in the motor cortex, the supplementary motor areas, and the cerebellum play a crucial role in rhythmic timing, such as feet and finger tapping to music (8). Rhythm processing could be selectively impaired without deficits in melody perception, suggesting the presence of a neural system specialized for rhythm (9). The left cerebral hemisphere is mainly involved in rhythm processing, but dysfunction in either the left or right hemisphere compromises tapping of rhythmic patterns from short musical pieces. A functional MRI study showed that tones spaced evenly at integer ratios (such as 1:2 and 1:3) evoked activation in the left premotor area and the right cerebellum, and tones spaced at complex ratios (such as 1:2.5) elicited activation in the right hemisphere, including the right premotor area, with bilateral activation in the cerebellum (10). The prefrontal cortex has extensive connections to all areas involved in music perception, processing, and generation.
In addition, the prefrontal cortex plays a crucial role in controlling executive function and motivation, both of which are critical for successful participation in a musical band. Executive function is defined as an ability to plan, prioritize, and initiate action. It also involves the ability to flexibly shift between tasks and inhibit automated responses for the sake of more effortful yet more important and optimal actions, such as forgoing immediate rewards for a delayed reward of playing well in a band. High levels of motivation are important in order to successfully play in a band. Apathy is a profound lack of interest and indifference, diminished motivation, loss of initiation, and poor persistence. Frontal lobe dysfunction is known to cause both executive dysfunction and apathy. Thus, stimulation of the DLPFC is an attractive target with a potential to improve multiple cognitive and behavioral processes critical in music recognition and playing.
Executive function improved in our patient as demonstrated by improvement in his processing speed-set shifting (Trail-Making Test, Part B) and complex attention (Conners’ Continuous Performance Test percent commission errors). This finding is consistent with other studies of rTMS stimulation of the DLPFC demonstrating improvement in various domains of executive function with similar stimulation parameters and number of stimulations (11–13). Enhanced executive function could have facilitated our patient’s improvement in his band performance.
Apathy improved significantly in our patient as measured by the Apathy Evaluation Scale. It is noteworthy that even after 12 rTMS sessions, his score of 47 would still be considered clinically significant apathy. This improvement in motivation is consistent with findings from other studies of neuromodulation and could be a key mediator in the improvement in our patient’s musical perception (14, 15).
Although we did not objectively test the patient’s pitch recognition, similar findings have been reported in other neuromodulation studies. In one study, suppressing the left supramarginal gyrus function using cathodal transcranial direct current stimulation led to a deterioration in pitch recognition ability (16). In another study of rTMS in 27 healthy participants, the left supramarginal gyrus was involved in the retention phase of pitch memory (17).
Conclusions
Brain activity responsible for music rhythm and beat is complex. In the patient in the above case, improvement of music rhythm with DLPFC stimulation could have been due to the extensive neuronal networks of the DLPFC to various brain areas responsible for rhythm perception. Moreover, the established enhancing effects of DLPFC stimulation on executive function and motivation could also have contributed to the improvement in music rhythm.
1 : Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement 2011; 7:532–539Crossref, Medline, Google Scholar
2 : The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry 1987; 48:314–318Medline, Google Scholar
3 : Relationships between parts A and B of the Trail Making Test. J Clin Psychol 1987; 43:402–409Crossref, Medline, Google Scholar
4 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
5 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
6 : Continuous Performance Test performance in a normative epidemiological sample. J Abnorm Child Psychol 2003; 31:555–562Crossref, Medline, Google Scholar
7 : Testing for causality with transcranial direct current stimulation: pitch memory and the left supramarginal gyrus. Neuroreport 2006; 17:1047–1050Crossref, Medline, Google Scholar
8 : Timing functions of the cerebellum. J Cogn Neurosci 1989; 1:136–152Crossref, Medline, Google Scholar
9 : Boundaries of separability between melody and rhythm in music discrimination: a neuropsychological perspective. Q J Exp Psychol A 1993; 46:301–325Crossref, Medline, Google Scholar
10 : Neural representation of a rhythm depends on its interval ratio. J Neurosci 1999; 19:10074–10081Crossref, Medline, Google Scholar
11 : Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol 2003; 114:1125–1132Crossref, Medline, Google Scholar
12 : Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology 2002; 58:1288–1290Crossref, Medline, Google Scholar
13 : Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci 2005; 229–230:157–161Crossref, Medline, Google Scholar
14 : Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: a double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res 2018; 261:312–318Crossref, Medline, Google Scholar
15 : The efficacy of high-frequency repetitive transcranial magnetic stimulation for improving apathy in chronic stroke patients. Eur Neurol 2017; 78:28–32Crossref, Medline, Google Scholar
16 : Testing for causality with transcranial direct current stimulation: pitch memory and the left supramarginal gyrus. Neuroreport 2006; 17:1047–1050Crossref, Medline, Google Scholar
17 : Anodal transcranial direct current stimulation over the supramarginal gyrus facilitates pitch memory. Eur J Neurosci 2013; 38:3513–3518Crossref, Medline, Google Scholar



