A Neuropsychiatric and Neuroimaging Study of Unilateral and Bilateral Striatal Ischemic Lesions
Abstract
Objective:
Neuropsychiatric disorders after striatal territory stroke have not been studied systematically. The investigators aimed to study the spectrum of cognitive and behavioral disorders following striatal infarcts.
Methods:
Different aspects of cognitive functions, including executive, frontal lobe, memory, visuospatial, language, and semantic processing, were assessed among patients with striatal infarct. Structural MRI data sets were obtained 3 months after stroke to delineate affected territories of the striatum. MRIcroGL software was used to acquire multiple layers of images, generate volume renderings, and draw volumes of interest. To determine the brain locus most frequently affected in patients with distinct cognitive disorders, ischemic area distributions in patients with cognitive dysfunction versus those without cognitive impairment were contrasted.
Results:
Among 60 patients in this study, six different striatal infarction types were significantly associated with seven different cognitive categories (p<0.001). Unilateral caudate lesion was characterized by attention, planning, and executive disorders (38%), and unilateral lentiform infarct was characterized by executive (36%) and frontal (36%) dysfunctions. Bilateral caudate infarcts caused impairments in frontal and executive functions (75%), as well as in autobiographical (50%) and episodic (50%) memory. In those with bilateral caudate plus lentiform infarcts, all components of frontal and executive functions were dramatically impaired. The anteromedial striatum was affected more frequently in patients with language impairment compared with patients with other types of cognitive dysfunction (p<0.001).
Conclusions:
Following striatal stroke, a wide range of frontal-like cognitive impairments occurred, along with impaired working memory, declarative memory, executive function, speech fluency, and motor function.
The striatum comprises the caudate nucleus and the lentiform nucleus, which are especially well developed in humans. The putamen is located medial to the cortex of the insula and surrounded laterally by the external capsule, medially by the lateral medullary lamina of the globus pallidus, and superiorly by the centrum semiovale (1, 2). Medial and lateral parts of the globus pallidus (paleostriatum) lie medial to the putamen and together with it compose the lentiform nucleus. Basal ganglia have been shown to receive topographically organized projections from various regions of the cerebral cortex (3), including the temporal lobe (4), parietal lobe (5), and brainstem (6). In addition, many afferent projections come from the frontal lobe, suggesting an important functional fronto-striatal interaction (among the medial, ventral, and dorsolateral prefrontal cortex; frontal pole; and presupplementary motor areas) (7, 8). These rich interconnections appear to play a key role in various functions associated with the limbic, oculomotor, motor, and cognitive systems. Not only do they support the idea that the basal ganglia are involved in habitual or automatic movements but also in cognition, possibly working in the same manner for both domains (9–11). Various studies have shown that damage to the basal ganglia is involved in a wide range of neuropsychiatric disorders.
The striatum is supplied by the lenticulostriate arteries, which are branches of the M1 segment of the middle cerebral artery (12). Different types of vascular cognitive deficits may occur among persons with a unilateral striatum lesion, and bilateral stroke lesions of the striatum can cause severe cognitive and behavioral disorders (13–15). Although considerable literature exists on this topic, the role of the striatum in cognitive and behavioral functions after stroke is not well defined. Here, we focused systematically on cognitive and behavioral functions, as well as MRI correlates, to understand how different types of striatal stroke affect cognition and behavior.
Methods
Patients who were admitted to our stroke center were studied both prospectively and longitudinally. Between January 1, 2012, and January 30, 2019, a total of 4,500 patients with ischemic stroke were recruited and prospectively entered into the Ege Stroke Registry (16). After exclusion criteria were applied, among 2,550 patients with anterior circulation stroke, 40 (1.6%) patients with unilateral lesion of the striatum and 20 (0.8%) with bilateral lesions were included in the study. The initial MRI was performed in all cases within 1 week after ictus and 3 months after stroke. Patient selection was based on the initial MRI performed during the first week. Striatal infarcts are defined as lesions involving the caudate nucleus, putamen, and pallidum that are at least 30 mm long and 10 mm wide without involvement of the capsula interna and overlying cerebral cortex (13–15). All patients had a standardized evaluation for their symptoms, and no previously diagnosed conditions affected their cognitive and behavioral functions. Prospectively recorded variables included age, gender, previous stroke, stroke risk factors, blood pressure, clinical findings, etiological subtypes, pathogenesis, and topography of lesions on diffusion-weighted imaging or fluid-attenuated inversion recovery (FLAIR) MRI. Vascular and cardiac structures were examined by using noninvasive modalities, such as carotid-vertebral and transcranial Doppler, MR angiography or computerized tomography angiography, two-dimensional echocardiography, and 24-hour electrocardiography (Holter) monitoring. The cause of stroke was assessed according to the criteria described previously as large-artery disease, small-artery disease, cardioembolic, other, or unknown (17). The study was approved by the ethics committee of the Ege University Medical Center review board, and written informed consent was obtained from all participants or their relatives.
Imaging Acquisition and Analysis
MRI was performed with 1.5- and 3.0-Tesla scanners (Siemens Sonata, Siemens Medical Solutions, Erlangen, Germany). MRI scanners consisted of axial T1-weighted and T2-weighted spin-echo, T2 FLAIR, and diffusion-weighted imaging. Two radiologists, blinded to each other’s decisions, visually identified and sequentially outlined lesion locations on diffusion-weighted imaging, FLAIR, and chronic T2-weighted images. Clinically relevant regions characterized by increased signal intensity on diffusion-weighted imaging and decreased intensity on the apparent diffusion coefficient maps were considered as acute infarct. Regions with increased signal intensity on follow-up T2-weighted images within the initially abnormal area on diffusion-weighted imaging were considered as the final infarct. Symmetric signal loss in the globus pallidum (most likely calcification), flow void artifact of the pial blood vessels, and intracerebral lesions with a hemorrhagic component were ruled out. On the basis of MRI findings, the lesion locations were classified as caudate, lenticular (putamen and pallidum), and combined (caudate plus lenticular). Patients with bilateral lesions were categorized in the following three groups: bilateral caudate (N=4), bilateral lenticular (N=11), and bilateral caudate plus lentiform (N=5) (Figure 1A).
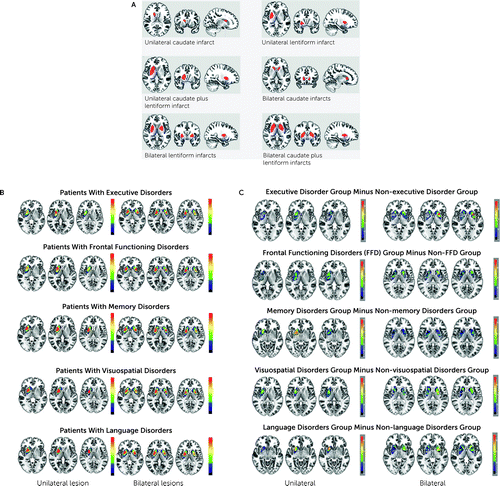
FIGURE 1. Pattern of unilateral and bilateral striatal infarcts in 60 patientsa
aIndividual infarct areas on axial, sagittal, and coronal sections were plotted individually onto the standard Montreal Neurological Institute 152 template (MRIcroGL software was used; https://www.mccauslandcenter.sc.edu/mricrogl/home). Panel A shows that the bilateral anterior striatum was most frequently affected in patients with executive disorders compared with patients with other cognitive and behavioral disorders (the maximum overlap was 75%, corresponding to N=12 of 16 patients). Panel B shows the overlap maps of patients with unilateral and bilateral striatal infarcts. Unilateral and bilateral ischemic areas were overlapped according to distinct cognitive disorders. Unilateral left lesions were flipped for this analysis (maximum overlapping is shown in red; minimum overlapping is shown in blue). Panel C shows the subtraction plot results. Anteromedial striatum (putamen plus caudate nuclei) was mostly affected in patients with frontal-functioning disorders (the maximum overlap was 76%, corresponding to N=13 of 17 patients with bilateral lesions). Bilateral anteromedial striatum (putamen) was most frequently affected in patients with memory disorders versus other cognitive and behavioral disorders (the maximum overlap was 67%, corresponding to N=10 of 15 patients). In patients with visuospatial disorders, the anteromedial striatum was damaged more frequently than were other striatal areas (the maximum overlap was 77%, corresponding to N=10 of 13 patients). The anteromedial striatum, especially the left, was most frequently affected in patients with language disorders versus other cognitive or behavioral disorders (the maximum overlap was 69%, corresponding to N=9 of 13 patients).
Neuropsychological Assessment
As part of the study protocol, the neuropsychological assessment was performed by two neuropsychologists, with accreditation in neurocognitive science, 1 week after the patients’ stroke (7 days [SD=2]) and again 3 months after stroke (90 days [SD=10]). Different aspects of cognitive functions have been assessed and classified into seven broad categories on the basis of similarity of addressed tasks and clusters of cross-references (18–21). First, global mental assessment was conducted with the Mini-Mental State Examination. Second, executive functioning was evaluated by using the Trail-Making Test (Parts A and B) and Stroop test (name color print of color and noncolor words). Third, frontal functioning was assessed with the Wisconsin Card Sorting Test (WCST), including measures of categories achieved, correct responses, errors, perseverative response, and failure to maintain. Fourth, autobiographical memory included the five most important personal events of patients’ lives; episodic memory was assessed with the Rey Auditory Verbal Learning Test, including measures of immediate free recall and delayed free recall for short- and long-term memory; semantic memory was evaluated with the Controlled Oral Word Association test; and visuospatial memory was assessed with the Rey–Osterrieth Complex Figure test. Fifth, visuospatial neglect assessment included the Bells Test, which involves encircling bells embedded within distractors (for example, houses and horses); visuospatial disorientation was tested with the Benton’s Judgment of Line Orientation test; and motor neglect and visual extinction were also assessed. Sixth, language skills, such as verbal fluency, comprehension, reading, writing, and calculation, were tested with the Turkish language version of the Ege Aphasia Test. Seventh, semantic processing was evaluated with the Pyramids and Palm Trees test (22). A z-score (standard score) of each patient’s cognitive test point was calculated, and patients with a z-score of 2.0 above or below the mean were considered as having pathological stroke. To determine patients’ prestroke cognitive status, we conducted an informant interview on the basis of current criteria for dementia and validated by reviewing the records of their primary care physician or records in Turkey’s National Health Information System. Previously, no striatal stroke patients were diagnosed with dementia. All the above assessments, means and standard deviations, scores, and associated references (14, 16) are described in detail in the online supplement.
Neuroanatomical Data Acquisition, Normalization, and Lesion Drawing
Structural MRI data sets were acquired 3 months after stroke as part of standard care. In particular, high-resolution three-dimensional T1-weighted sequences were retained for the purposes of this study (3.0 Tesla Siemens scanner). The imaging parameters were as follows: 1-mm isometric voxel; reaction time=1.7 s; echo time=2.54 ms; inversion time=0.92 s; and field of view=256×256 mm. To normalize individual imaging data sets spatially, we used SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12), implemented in MATLAB (release 2014b, MathWorks, Natick, Mass.), with standard parameters. At this stage, all 3-month normalized MRIs (Montreal Neurological Institute 152 brain template) were manually checked to ensure that all normalizations were of sufficient quality. We used MRIcroGL software (http://people.cas.sc.edu/rorden/mricron/install.html) to acquire multiple layers of images, generate volume renderings, and draw volumes of interest. We contoured by hand the ischemic areas and transformed them into binarized images by using MRIcroGL software. This work yielded 14 volumes of interest (Figure 1B).
Subtraction Plots
To determine which striatal territories were the most frequently affected by stroke in patients showing different categories of neurocognitive deficits, we first overlapped the damaged area in the patients showing neurocognitive deficits and in those not showing neurocognitive deficits. Next, we contrasted both overlap maps using the MRIcron subtraction-plot function. The infarct area maps of patients with a left infarct were inverted for this analysis, and the others are described in the following (Figure 1C).
Statistical Analysis
Suitability of numerical variables to normal distribution was examined by using the Kolmogorov–Smirnov test. Numerical variables are presented as means and standard deviations. Categorical variables are presented as frequencies and percentages. Chi-square tests were used for comparisons of categorical variables. Analysis of variance was performed for more than two group comparisons. To combine tasks, we calculated z-scores for neuropsychological tests at baseline for all patients. A z-score of 2.0 above or below the mean indicating a 97.7% probability of the standard deviation from the mean was considered abnormal. All statistical analyses were conducted with SPSS 22.0 for Windows (SPSS, Chicago).
Results
The patient population comprised 31 (52%) females and 29 males (48%), with a mean age of 66.4 years (SD=11.9; interquartile range 40–89 years) and a mean educational level of 8.6 years (SD=2.8). All patients were right-handed. The ischemic lesion was on the left side in 23 (38%) patients, on the right side in 17 (28%) patients, and on both sides in 20 (33%) patients. The patients’ sociodemographic and clinical data are summarized in Table 1.
| Characteristic | Striatal infarcts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unilateral lesion | Bilateral lesions | |||||||||||
| Caudate (N=8) | Lentiform (N=22) | Caudate plus lentiform (N=10) | Caudate (N=4) | Lentiform (N=11) | Caudate plus lentiform (N=5) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age in years | 65.3 | 11.9 | 70.9 | 12.1 | 66.3 | 10.9 | 70.5 | 10.7 | 61.7 | 10.0 | 55.8 | 11.9 |
| Education level (years) | 8.1 | 3.6 | 7.9 | 3.26 | 8.3 | 2.4 | 7.5 | 3.1 | 7.4 | 3.1 | 9.0 | 7.9 |
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Sex (male) | 4 | 50.0 | 11 | 50.0 | 4 | 40.0 | 2 | 50.0 | 4 | 36.0 | 4 | 80.0 |
| Cause of stroke | ||||||||||||
| Large-artery disease | 1 | 13.0 | 5 | 23.0 | 2 | 20.0 | 1 | 25.0 | 4 | 36.0 | 3 | 60.0 |
| Cardioembolism | 1 | 13.0 | 2 | 9.0 | 1 | 10.0 | 0 | — | 1 | 9.0 | 0 | — |
| Small-artery disease | 6 | 75.0 | 11 | 50.0 | 4 | 40.0 | 1 | 25.0 | 4 | 36.0 | 0 | — |
| Other | 0 | — | 1 | 5.0 | 1 | 10.0 | 0 | — | 0 | — | 1 | 20.0 |
| Unknown | 0 | — | 3 | 14.0 | 2 | 20.0 | 2 | 50.0 | 2 | 18.0 | 1 | 20.0 |
| Somnolence | 0 | — | 3 | 14.0 | 5 | 50.0 | 1 | 25.0 | 6 | 55.0 | 4 | 80.0 |
| Confusion | 2 | 25.0 | 1 | 5.0 | 3 | 30.0 | 2 | 50.0 | 6 | 55.0 | 5 | 100.0 |
| Disinhibition | 0 | — | 0 | — | 1 | 10.0 | 0 | — | 4 | 36.0 | 2 | 40.0 |
| Abulia | 2 | 25.0 | 7 | 32.0 | 4 | 40.0 | 1 | 25.0 | 6 | 55.0 | 4 | 80.0 |
| Nonfluent speech | 0 | — | 2 | 9.0 | 4 | 40.0 | 2 | 50.0 | 6 | 55.0 | 5 | 100.0 |
| Motor deficit | ||||||||||||
| Facio-brachial | 1 | 13.0 | 7 | 32.0 | 5 | 50.0 | 1 | 25.0 | 2 | 18.0 | 1 | 20.0 |
| Facio-brachio-crural | 0 | — | 7 | 32.0 | 5 | 50.0 | 1 | 25.0 | 5 | 45.0 | 3 | 60.0 |
| Bilateral | 0 | — | 0 | — | 0 | — | 2 | 50.0 | 4 | 36.0 | 1 | 20.0 |
| Dysarthria | 2 | 25.0 | 5 | 23.0 | 5 | 50.0 | 2 | 50.0 | 8 | 73.0 | 5 | 100.0 |
| Parkinsonism | 0 | — | 3 | 14.0 | 4 | 40.0 | 1 | 25.0 | 4 | 36.0 | 3 | 60.0 |
| Chorea | 0 | — | 1 | 5.0 | 1 | 10.0 | 0 | — | 1 | 9.0 | 1 | 20.0 |
| Dystonia | 0 | — | 3 | 14.0 | 4 | 40.0 | 1 | 25.0 | 5 | 46.0 | 3 | 60.0 |
| Tremor | 1 | 13.0 | 4 | 18.0 | 4 | 40.0 | 1 | 25.0 | 5 | 46.0 | 3 | 60.0 |
| Ataxia | 1 | 13.0 | 2 | 9.0 | 3 | 30.0 | 2 | 50.0 | 4 | 36.0 | 4 | 80.0 |
TABLE 1. Baseline demographic and clinical characteristics of patients with unilateral and bilateral lesions of the striatum after stroke
In the first week of stroke, 65% (N=13) of patients with bilateral lesions had a high level of confusion compared with 15% (N=6) of those with unilateral infarct (p<0.001). Somnolence was more frequent among patients with bilateral striatal infarct (55%, N=11) than among those with unilateral infarct (20%, N=8) (p<0.02). Motor deficits were generally associated with unilateral lesions (100%, N=20), and faciobrachial weakness was present in 13 (33%) patients with a unilateral lesion and in four (20%) patients with bilateral lesions. Facio-brachio-crural motor disturbance was experienced by 12 (30%) patients with a unilateral lesion and nine (45%) patients with bilateral lesions.
Unilateral Caudate Infarct
Unilateral caudate infarct was characterized by disorders in executive attention (38%), short-term working memory (38%), and semantic memory (25%). Abnormal findings of dysarthria (25%) and abulia (25%) were each present in two patients (Table 2).
| Neuropsychological assessment | Unilateral lesion | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caudate infarct (N=8) | Lentiform infarct (N=22) | Caudate plus lentiform infarct (N=10) | ||||||||||||||||
| 1 week | 3 months | 1 week | 3 months | 1 week | 3 months | |||||||||||||
| N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | |
| Global mental impairment | ||||||||||||||||||
| MMSE (score ≤24) | 2 | 25.0 | −2.3 | 0 | — | −1.6 | 8 | 36.0 | −2.5 | 2 | 9.0 | −2.3 | 6 | 60.0 | −2.9 | 2 | 20.0 | −2.4 |
| Executive-functioning impairment | ||||||||||||||||||
| Stroop test | ||||||||||||||||||
| Name color print of noncolor words | 2 | 25.0 | −2.4 | 0 | — | 7 | 32.0 | −2.5 | 2 | 9.0 | −2.3 | 5 | 50.0 | −2.7 | 2 | 20.0 | −2.5 | |
| Name color print of color words | 3 | 38.0 | −2.3 | 1 | 13.0 | −2.1 | 8 | 36.0 | −2.7 | 3 | 14.0 | −2.5 | 6 | 60.0 | −2.8 | 3 | 30.0 | −2.6 |
| Trail-Making Test | ||||||||||||||||||
| Part A | 2 | 25.0 | −2.5 | 0 | — | 6 | 27.0 | −2.6 | 2 | 9.0 | −2.7 | 4 | 40.0 | −2.9 | 1 | 10.0 | −2.6 | |
| Part B | 3 | 38.0 | −2.4 | 1 | 13.0 | −2.2 | 7 | 32.0 | −2.6 | 3 | 14.0 | −2.4 | 5 | 50.0 | −2.7 | 2 | 20.0 | −2.5 |
| Frontal-functioning impairment (WCSTb) | ||||||||||||||||||
| Categories achieved | 7 | 88.0 | −2.5 | 8 | 100.0 | −2.3 | 17 | 77.0 | −2.5 | 19 | 86.0 | −2.2 | 3 | 30.0 | −2.8 | 7 | 70.0 | −2.5 |
| Correct responses | 6 | 75.0 | −2.3 | 7 | 88.0 | −2.1 | 14 | 64.0 | −2.6 | 18 | 82.0 | −2.2 | 3 | 30.0 | −2.8 | 4 | 40.0 | −2.7 |
| Errors | 1 | 13.0 | −2.3 | 0 | — | 8 | 36.0 | −2.8 | 2 | 9.0 | −2.4 | 7 | 70.0 | −2.9 | 3 | 30.0 | −2.4 | |
| Perseverative responses | 1 | 13.0 | −2.3 | 1 | 13.0 | −2.1 | 8 | 36.0 | −2.4 | 2 | 9.0 | −2.3 | 6 | 60.0 | −2.7 | 2 | 20.0 | −2.6 |
| Failure to maintain | 1 | 13.0 | −2.1 | 0 | — | 5 | 23.0 | −2.5 | 2 | 9.0 | −2.4 | 5 | 50.0 | −2.8 | 3 | 30.0 | −2.5 | |
| Memory impairment | ||||||||||||||||||
| Autobiographical memory | 1 | 13.0 | −2.1 | 0 | — | 1 | 5.0 | −2.3 | 0 | — | 3 | 30.0 | −2.8 | 1 | 10.0 | −2.4 | ||
| Episodic memory (time, place, and feature) | 2 | 25.0 | −2.4 | 0 | — | 3 | 14.0 | −2.5 | 0 | — | 6 | 60.0 | −2.8 | 2 | 20.0 | −2.5 | ||
| Semantic memory (COWA) | 2 | 25.0 | −2.3 | 1 | 13.0 | −2.3 | 5 | 23.0 | −2.6 | 1 | 5.0 | −2.3 | 5 | 50.0 | −2.7 | 2 | 20.0 | −2.4 |
| Short-term memory (AVLT) | 3 | 38.0 | −2.4 | 1 | 13.0 | −2.5 | 5 | 23.0 | −2.4 | 1 | 5.0 | −2.2 | 6 | 60.0 | −2.9 | 3 | 30.0 | −2.5 |
| Long-term memory (AVLT) | 2 | 25.0 | −2.3 | 0 | — | 3 | 14.0 | −2.4 | 0 | — | 3 | 20.0 | −2.7 | 2 | 20.0 | −2.6 | ||
| Visuospatial memory (ROCFT) | 0 | — | 0 | — | 2 | 9.0 | −2.1 | 0 | — | 3 | 30.0 | −2.6 | 0 | — | ||||
| Visuospatial impairment | ||||||||||||||||||
| Spatial disorientation (BJLO) | 1 | 13.0 | −2.6 | 0 | — | 3 | 14.0 | −2.6 | 0 | — | 3 | 30.0 | −2.7 | 1 | 10.0 | −2.3 | ||
| Spatial neglect (line bisection task) | 0 | — | 0 | — | 0 | — | 0 | — | 1 | 10.0 | −2.5 | 0 | — | |||||
| Motor neglect | 0 | — | 0 | — | 0 | — | 0 | — | 1 | 10.0 | −2.5 | 0 | — | |||||
| Visual extinction | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Language impairment | ||||||||||||||||||
| Nonfluent speech | 0 | — | 0 | — | 2 | 9.0 | −2.6 | 1 | 5.0 | −2.4 | 4 | 40.0 | −2.9 | 2 | 20.0 | −2.5 | ||
| Comprehension | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Acalculia | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Agraphia | 0 | — | 0 | — | 0 | — | 0 | — | 1 | 10.0 | −2.3 | 0 | — | |||||
| Alexia | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Semantic-processing impairment | ||||||||||||||||||
| Verbal/word comprehension (PPT) | 1 | 13.0 | −2.3 | 0 | — | 4 | 18.0 | −2.5 | 1 | 5.0 | −2.2 | 4 | 40.0 | −2.8 | 1 | 10.0 | −2.5 | |
| Nonverbal/picture comprehension (PPT) | 0 | — | 0 | — | 5 | 23.0 | −2.6 | 1 | 5.0 | −2.3 | 3 | 30.0 | −2.7 | 2 | 20.0 | −2.4 | ||
TABLE 2. Neuropsychological impairments at the cognitive domain levels and z-scores of patients with unilateral infarcts of the striatum, by time of assessmenta
Unilateral Lentiform Infarct
Unilateral lentiform infarct mainly caused executive-functioning (36%), frontal-functioning (36%), and various types of memory disorders, including episodic (14%) and semantic (23%) memory impairments. Visuospatial memory disturbance was observed in two (9%) patients with a right-sided lesion (Table 2).
Unilateral Caudate Plus Lentiform Infarct
In the unilateral caudate plus lentiform infarct group, in addition to frontal and executive disorders, patients experienced other cognitive disorders, including spatial disorientation (30%), neglect (10%), nonfluent speech (40%), and semantic-processing disorders (40%) (Table 2 and Figure 2).
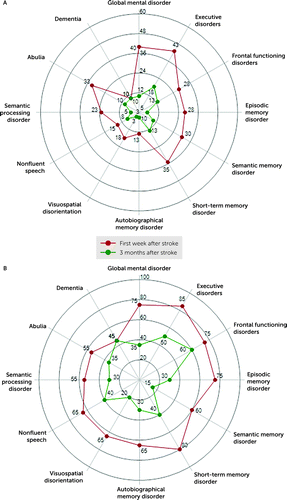
FIGURE 2. Frequency (%) of 12 cognitive and behavioral disorders among 60 patients with unilateral and bilateral lesions of the striatum during the first week and 3 months after strokea
aFrequency of 12 cognitive and behavioral disorders among 60 patients with unilateral (A) and bilateral (B) lesions of the striatum during the first week and 3 months after stroke. For further details, see Figure S2 in the online supplement.
Bilateral Caudate Infarcts
Among patients with bilateral caudate infarcts, frontal and executive functioning were disrupted, and autobiographical memory (50%), episodic memory (50%), short-term memory (75%), speech fluency (50%), and semantic processing (50%) were also impaired (Table 3).
| Neuropsychological assessment | Bilateral lesions | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilateral caudate infarcts (N=4) | Bilateral lentiform infarcts (N=11) | Bilateral caudate plus lentiform infarcts (N=5) | ||||||||||||||||
| 1 week | 3 months | 1 week | 3 months | 1 week | 3 months | |||||||||||||
| N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | N | % | z-score | |
| Global mental impairment | ||||||||||||||||||
| MMSE (score ≤24) | 2 | 50.0 | −2.5 | 1 | 25.0 | −2.4 | 8 | 73.0 | −2.8 | 3 | 27.0 | −2.4 | 5 | 100.0 | −3.2 | 3 | 60.0 | −2.9 |
| Executive-functioning impairment | ||||||||||||||||||
| Stroop test | ||||||||||||||||||
| Name color print of noncolor words | 2 | 50.0 | −2.7 | 1 | 25.0 | −2.4 | 8 | 73.0 | −2.8 | 4 | 36.0 | −2.4 | 5 | 100.0 | −3.3 | 3 | 60.0 | −2.9 |
| Name color print of color words | 3 | 75.0 | −2.4 | 2 | 50.0 | −2.5 | 9 | 82.0 | −2.9 | 4 | 36.0 | −2.6 | 5 | 100.0 | −3.1 | 4 | 80.0 | −2.8 |
| Trail-Making Test | ||||||||||||||||||
| Part A | 2 | 50.0 | −2.8 | 1 | 25.0 | −2.4 | 7 | 64.0 | −3.1 | 2 | 18.0 | −2.8 | 4 | 80.0 | −3.4 | 4 | 80.0 | −3.0 |
| Part B | 3 | 75.0 | −2.9 | 2 | 50.0 | −2.3 | 8 | 73.0 | −2.8 | 4 | 36.0 | −2.4 | 5 | 100.0 | −3.3 | 4 | 80.0 | −3.1 |
| Frontal-functioning impairment | ||||||||||||||||||
| Wisconsin Card Sorting Test | ||||||||||||||||||
| Categories achieved | 2 | 50.0 | −2.8 | 3 | 75.0 | −2.6 | 3 | 27.0 | −2.7 | 4 | 36.0 | −2.4 | 5 | 100.0 | −3.2 | 4 | 80.0 | −2.8 |
| Correct responses | 3 | 75.0 | −2.6 | 3 | 75.0 | −2.4 | 6 | 55.0 | −2.7 | 7 | 64.0 | −2.6 | 5 | 100.0 | −3.1 | 4 | 80.0 | −2.4 |
| Errors | 3 | 75.0 | −2.9 | 2 | 50.0 | −2.6 | 9 | 82.0 | −3.1 | 7 | 64.0 | −2.8 | 5 | 100.0 | −3.3 | 4 | 80.0 | −2.9 |
| Perseverative responses | 3 | 75.0 | −2.9 | 2 | 50.0 | −2.7 | 9 | 82.0 | −2.8 | 6 | 55.0 | −2.3 | 4 | 80.0 | −3.1 | 3 | 60.0 | −2.8 |
| Failure to maintain | 2 | 50.0 | −2.8 | 2 | 50.0 | −2.6 | 8 | 73.0 | −2.9 | 7 | 64.0 | −2.5 | 5 | 100.0 | −2.9 | 4 | 80.0 | −2.7 |
| Memory impairment | ||||||||||||||||||
| Autobiographical memory | 2 | 50.0 | −2.4 | 1 | 25.0 | −2.2 | 7 | 64.0 | −2.6 | 3 | 27.0 | −2.4 | 4 | 80.0 | −2.9 | 2 | 40.0 | −2.7 |
| Episodic memory (time, place, and feature) | 2 | 50.0 | −2.7 | 0 | 0 | −1.44 | 8 | 73.0 | −2.8 | 3 | 27.0 | −2.5 | 5 | 100.0 | −3.2 | 3 | 60.0 | −3.0 |
| Semantic memory (COWA) | 2 | 50.0 | −2.6 | 1 | 1.0 | −2.3 | 6 | 55.0 | −2.9 | 2 | 18.0 | −2.7 | 4 | 80.0 | −3.2 | 3 | 60.0 | −2.9 |
| Short-term memory (AVLT) | 3 | 75.0 | −2.7 | 1 | 1.0 | −2.4 | 8 | 73.0 | −32 | 3 | 27.0 | −2.9 | 5 | 100.0 | −3.4 | 4 | 80.0 | −3.1 |
| Long-term memory (AVLT) | 2 | 25.0 | −2.4 | 0 | 0 | 6 | 55.0 | −2.8 | 2 | 18.0 | −2.8 | 3 | 60.0 | −3.3 | 2 | 40.0 | −3.0 | |
| Visuospatial memory (RCFT) | 1 | 25.0 | −2.4 | 0 | — | 4 | 36.0 | −2.9 | 1 | 9.0 | −2.6 | 3 | 60.0 | −2.8 | 2 | 40.0 | −2.6 | |
| Visuospatial impairment | ||||||||||||||||||
| Spatial disorientation (BJLO) | 2 | 50.0 | −2.4 | 0 | — | 6 | 55.0 | −2.7 | 2 | 18.0 | −2.3 | 5 | 100.0 | −3.1 | 2 | 40.0 | −2.3 | |
| Spatial neglect (line bisection task) | 0 | — | 0 | — | 1 | 9.0 | −2.6 | 0 | — | 1 | 20.0 | −2.5 | 0 | — | ||||
| Motor neglect | 0 | — | 0 | — | 0 | — | 0 | — | 1 | 20.0 | −2.5 | 0 | — | |||||
| Visual extinction | 0 | — | 0 | — | 0 | — | 0 | — | 0 | 0 | 0 | — | ||||||
| Language impairment | ||||||||||||||||||
| Nonfluent speech | 2 | 50.0 | −2.5 | 1 | 25.0 | −2.8 | 6 | 55.0 | −2.6 | 3 | 27.0 | −2.5 | 5 | 100.0 | −3.1 | 4 | 80.0 | −2.9 |
| Comprehension | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Acalculia | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | ||||||
| Agraphia | 0 | — | 0 | — | 0 | — | 0 | — | 2 | 40.0 | 1 | 20.0 | −2.8 | |||||
| Alexia | 0 | — | 0 | — | 2 | 18.0 | −2.7 | 0 | — | 0 | — | 0 | — | |||||
| Semantic-processing impairment | ||||||||||||||||||
| Verbal/word comprehension (PPT) | 2 | 50.0 | −2.7 | 1 | 25.0 | −2.3 | 5 | 45.0 | −2.9 | 2 | 18.0 | −2.6 | 4 | 80.0 | −3.2 | 3 | 60.0 | −3.0 |
| Nonverbal/picture comprehension (PPT) | 1 | 25.0 | −2.6 | 0 | — | 3 | 27.0 | −2.8 | 1 | 9.0 | −2.5 | 2 | 40.0 | −2.9 | 2 | 40.0 | −2.8 | |
TABLE 3. Neuropsychological impairments at the cognitive domain levels and z-scores of patients with bilateral infarcts of the striatum, by time of assessmenta
Bilateral Lentiform Infarcts
Frontal and executive functioning were severely impaired among patients with bilateral lentiform infarcts (73%). Six patients experienced nonfluent speech and dysarthria. Almost all components of explicit memory were severely impaired in this patient group (Table 3).
Bilateral Caudate Plus Lentiform Infarcts
In patients with bilateral caudate plus lentiform infarcts, all components of frontal and executive functioning were dramatically impaired, and other cognitive domains, including spatial, memory, and language, were severely affected. Most of the patients (80%, N=4) showed dementia syndrome 3 months after stroke (Table 3).
Extrapyramidal Disorders
Different types of extrapyramidal motor disorders were present 3 months after stroke. Parkinsonism was observed mostly among patients with unilateral (40%) and bilateral lentiform (60%) infarcts. Dystonia and tremor were frequently seen among patients with unilateral caudate plus lentiform infarcts (40%) and bilateral caudate plus lentiform infarcts (60%) (Table 1).
Subtraction Plot Results
The bilateral anterior striatum, versus other striatal areas, was most frequently affected in patients with executive disorders (the maximum overlap was 75%, N=12 of 16 patients) (p<0.001). The anteromedial striatum was most frequently affected in patients with frontal disorders (the maximum overlap was 76%). Bilateral anteromedial striatum was most frequently damaged in patients with memory disorders versus other cognitive dysfunction (the maximum overlap was 67%) (p<0.01). The right anteromedial striatum was affected more frequently in patients with visuospatial impairment than in those with other cognitive impairments (the maximum overlap was 77%, corresponding to N=5 of 7 patients) (p<0.001). Anteromedial striatum was most frequently damaged in patients with language disorders compared with those with other cognitive or behavioral disorders (the maximum overlap was 69%) (p<0.001).
Discussion
The findings of this study help clarify neuropsychiatric dysfunctions of the striatum after stroke. Moreover, neuroanatomical analysis showed that lesions in the caudate nucleus and lentiform nucleus are likely to be associated with different motor and neuropsychological disorders.
First, we note that stroke-induced unilateral caudate lesions were associated with specific impairments in executive functions, attention, and short-term memory, suggesting a strong functional bond between the caudate nucleus and the prefrontal cortex (23–25). The deficits observed after bilateral caudate infarcts were more prominent than those observed after other infarcts examined and were strikingly similar to those found in frontal lobe dysfunction (26). The marked disturbance of executive functions following bilateral caudate involvement in stroke was specifically associated with diminished capacity to plan and organize personal strategies toward goal-directed actions. In addition, given the established roles of these brain areas in visuospatial processing (27) and cross-modal integration, respectively, bilateral damage to caudate regions may lead to visuospatial disorientation. Several studies have highlighted the role of basal ganglia in memory systems involving both relational learning and procedural learning (28). Consistent with this assertion, bilateral caudate lesions in particular have been associated with impairments in autobiographical memory, short-term memory, and learning, possibly related to a disruption in the network between the frontal cortex, anterior cingulate gyrus, and caudate nucleus.
In this study, lentiform involvement was the most frequent infarct type in the striatum. It is worth noting that abulia, characterized by loss of drive, expression, behavior, and speech output, was significantly increased in infarcts with putamen involvement. Numerous functional studies have addressed the possible association of the putamen with motor functions. Specifically, it has been proposed that the putamen contributes to movement preparation during self-initiated behavior (29). A study using positron emission tomography revealed significantly greater activation in the putamen and the anterior supplementary motor area during a paced self-initiated task (30). Dysarthria and nonfluent speech were other characteristics of lentiform involvement in stroke, especially among patients with bilateral lesions. These results are in line with those of lesion and neurofunctional imaging studies. Indeed, many investigators have reported speech-planning disorders after infarction or hemorrhage involving the left putamen (31). Some of the first neuroimaging studies of neural processes during speech revealed activation in language-associated regions and in the left striatum during word production, as well as repetition of single words or single syllables; damage in these areas may eventually contribute to the development of word-finding difficulties and speech fluency disorders (32). Of note, half of the patients with bilateral lentiform damage in our study experienced anarthria, supporting the view that the striatum is widely involved in motor speech and that these processes are managed by innervation from both cerebral hemispheres (33).
The WCST has been used as a neuropsychological test to assess cognitive flexibility after lentiform damage (34). Among patients with caudate and lentiform infarct in our study, set-shifting, which can be defined as the ability to change attention from one response set to another according to a change in task goals, was severely impaired, especially among individuals with bilateral lesions. Results from a more recent study (35) have suggested a significant increase of activity in the ventrolateral prefrontal cortex and caudate nucleus during the reception of negative feedback, indicating that a shift to a new response set is required, whereas the putamen was found to be significantly active together with the posterior prefrontal cortex and premotor cortex during matching after negative feedback, suggesting involvement in the execution of novel actions.
Caudate plus lentiform infarcts are uncommon compared with other infarct types in the striatum. Lentiform and caudate nuclei associated with the prefrontal lobe play an important role during performances related to executive function. These strategies include planning, inhibition, selective attention, and mental flexibility, as well as manipulation of information within working memory (36). The known roles of these areas include executive and mental processes for planning and organizing personal strategies toward goal-directed actions, and damage to these regions may lead to frontal dysfunction. In accordance with the known roles of the lentiform and caudate nuclei, we observed severe deterioration in frontal functions among the patients with unilateral infarcts and, especially, with bilateral lentiform plus caudate infarction.
In those with bilateral lentiform and lentiform plus caudate infarcts, working memory, which is important in the ability to hold and manipulate information, was significantly impaired. Autobiographical performance and declarative memory performance were also compromised among these patients, even if they differed in the locations of the lesions. In a meta-analysis, memory retrieval was shown to consistently activate striatal areas, including both the dorsal striatum in the left caudate and the ventral striatum (37). This finding appears to validate the hypothesis that a lesion damaging the medial striatum may cause declarative memory disorders.
Recent findings have suggested that a likely interaction of the striatum with the prefrontal cortex plays a causal role in the goal-directed retrieval and selection of semantic information from memory (38). Semantic memory refers to knowledge of facts, concepts, and word meanings that are independent of a specific encoding context and that may be stored in a distributed neocortical representation (39). Consistent with this model, observations with our patients with lentiform and caudate lesions revealed that the lesion locations associated with semantic verbal memory and fluency impairments matched specifically with areas known to be engaged in cognitive control during semantic memory tasks.
We noted that bilateral lesions of the lentiform plus caudate nuclei were associated with a severe decrease in cognitive functions, which may eventually lead to the development of dementia syndrome. Dementia identified in this study was possibly associated with bilateral dopamine depletion within the striatum and the terminal distribution of its cortical afferents; dysregulation of the network emanating from the mesolimbic and mesocortical dopaminergic systems, as well as nonstriatal and nondopaminergic systems, is a possible mechanism leading to dementia (40).
We note several limitations to our study. First, we used conventional neurological and neuropsychological tests instead of specific experimental manipulations, and we did not attempt to provide functional imaging data on underlying impaired mechanisms leading to cognitive disorders. Second, it is worth noting that we cannot entirely rule out the possibility that structures adjacent to stroke regions may have also been involved in the development of the impairments studied.
Conclusions
The findings of this study revealed several different cognitive and behavioral disorders that occur when different regions of the striatum are affected by ischemic events. Future studies are needed to reveal the precise neural mechanisms that are occurring in this part of the basal ganglia and that, when impaired, can cause neuropsychiatric disorders.
1. : The Human Brain: An Introduction to Its Functional Anatomy. St Louis, Mosby, 2002Google Scholar
2. Topography of cortico-striatal connections in man: anatomical evidence for parallel organization. Eur J Neurosci 2004; 20:1915–1922Crossref, Medline, Google Scholar
3. : Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007; 64:20–24Crossref, Medline, Google Scholar
4. : The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA 1996; 93:8683–8687Crossref, Medline, Google Scholar
5. : Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 1988; 8:4049–4068Crossref, Medline, Google Scholar
6. : The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 2010; 20:261–270Crossref, Medline, Google Scholar
7. : Frontal eye field efferents in the macaque monkey, I: subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol 1988; 271:473–492Crossref, Medline, Google Scholar
8. : Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol 1991; 312:43–67Crossref, Medline, Google Scholar
9. : The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12:366–375Crossref, Medline, Google Scholar
10. : Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol 1993; 3:950–957Crossref, Medline, Google Scholar
11. : Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 1990; 28:1021–1034Crossref, Medline, Google Scholar
12. : Diagnostic Cerebral Angiography. Philadelphia, Lippincott Williams & Wilkins, 1999Google Scholar
13. : The stroke syndrome of striatocapsular infarction. Brain 1991; 114:51–70Medline, Google Scholar
14. : Acute caudate vascular lesions. Stroke 1999; 30:100–108Crossref, Medline, Google Scholar
15. : Lenticulostriate infarction, in Manifestations of Stroke, vol 30. Edited by Paciaroni M, Agnelli G, Caso V, Bogousslavsky J. Basel, Front Neurol Neurosci Karger, 2012, pp 115–119Crossref, Google Scholar
16. : The Ege Stroke Registry: a hospital-based study in the Aegean Region, Izmir, Turkey. Cerebrovasc Dis 1998; 8:278–288Crossref, Medline, Google Scholar
17. : Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41Crossref, Medline, Google Scholar
18. : Contributions to Neuropsychological Assessment: A Clinical Manual, 2nd ed. Oxford, Oxford Universy Press, 1994Google Scholar
19. : Neuropsychological Assessment. Oxford, Oxford University Press, 1995Google Scholar
20. : Handbook of Normative Data for Neuropsychological Assessment, 2nd ed. Oxford, Oxford University Press, 2005Google Scholar
21. : Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford, Oxford University Press, 2006Google Scholar
22. : The Pyramids and Palm Trees Test: A Test of Semantic Access From Words And Pictures. Bury St Edmonds, United Kingdom, Thames Valley Test Company, 1992Google Scholar
23. : Neurological syndrome following bilateral damage to the head of the caudate nuclei. Ann Neurol 1987; 22:768–771Crossref, Medline, Google Scholar
24. : Neurobehavioral changes associated with caudate lesions. Neurology 1989; 39:349–354Crossref, Medline, Google Scholar
25. : Selective speech motor, syntax and cognitive deficits associated with bilateral damage to the putamen and the head of the caudate nucleus: a case study. Neuropsychologia 1998; 36:173–188Crossref, Medline, Google Scholar
26. : Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 2004; 10:525–537Crossref, Medline, Google Scholar
27. : Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Mov Disord 2009; 24:1193–1199Crossref, Medline, Google Scholar
28. : Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 1989; 9:1465–1472Crossref, Medline, Google Scholar
29. : Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1989; 85:119–146Crossref, Google Scholar
30. : Self-initiated versus externally triggered movements. Brain 2000; 123:1216–1228Crossref, Medline, Google Scholar
31. : Subcortical aphasia distinct profiles following left putaminal hemorrhage. Neurology 1995; 45:38–41Crossref, Medline, Google Scholar
32. : Left putaminal activation when speaking a second language: evidence from PET. Neuroreport 1994; 5:2295–2297Crossref, Medline, Google Scholar
33. : Brain regions involved in articulation. Lancet 1999; 353:1057–1061Crossref, Medline, Google Scholar
34. : Effects of different brain lesions on card sorting. Arch Neurol 1963; 9:90–100Crossref, Google Scholar
35. : Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 2001; 21:7733–7741Crossref, Medline, Google Scholar
36. : Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. Neuroimage 2010; 51:421–431Crossref, Medline, Google Scholar
37. : Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage 2011; 54:2446–2461Crossref, Medline, Google Scholar
38. : Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 2005; 47:907–918Crossref, Medline, Google Scholar
39. : The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci 2003; 4:310–322Crossref, Medline, Google Scholar
40. : Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 2001; 11:1136–1143Crossref, Medline, Google Scholar



