Possible Role of Parvalbumin Interneurons in Meditation and Psychiatric Illness
Abstract
Parvalbumin (PV) interneurons are present in multiple brain regions and produce complex influences on brain functioning. An increasing number of research findings indicate that the function of these interneurons is more complex than solely to inhibit pyramidal neurons in the cortex. They generate feedback and feedforward inhibition of cortical neurons, and they are critically involved in the generation of neuronal network oscillation. These oscillations, generated by various brain regions, are linked to perceptions, thought processes, and cognitive functions, all of which, in turn, influence human emotions and behavior. Both animal and human studies consistently have found that meditation practice results in enhancement in the effects of alpha-, theta-, and gamma-frequency oscillations, which may correspond to positive changes in cognition, emotion, conscious awareness, and, subsequently, behavior. Although the study of meditation has moved into mainstream neuroscience research, the link between PV interneurons and any role they might play in meditative states remains elusive. This article is focused primarily on gamma-frequency oscillation, which is generated by PV interneurons, to develop insight and perspective into the role of PV interneurons in meditation. This article also points to new and emerging directions that address whether this role of PV interneurons in meditation extends to a beneficial, and potentially therapeutic, role in the treatment of common psychiatric disorders, including schizophrenia.
Parvalbumin (PV) interneurons are the fast-spiking interneurons that generate gamma frequency and are involved in inhibiting the cortical pyramidal neurons that project to the striatal neurons, thus modulating important aspects of our motor skills and habitual learning. PV interneurons are highly vulnerable to stressors and have been implicated in many neuropsychiatric diseases. They require high energy to support a high metabolic activity and to safeguard from the glutamatergic excitation and stress (1). Since 1980, various writings and the findings of other researchers have generated increasing curiosity and inquiry in gamma oscillations generated by PV interneurons (2). Neural network modeling and theorizing postulates that the gamma oscillatory activity has its basis in the synaptic feedback interactions.
Specifically, gamma oscillation lies in the assemblies of brain excitatory and inhibitory (E-I) neurons, so there is a neural basis for gamma frequency and its amplitude (3). In some studies, gamma activity has been detected in long-term meditation practice (4). Nonetheless, in experienced meditators, the increase in the gamma oscillations as well as the alpha- and theta-frequency oscillations, corresponds to increased enhancement and effectiveness of numerous complex and psychologically adaptive traits, such as their mental and behavioral self-control and the display of (interpersonal) compassion, their ability to experience wellness and happiness, and their ability to achieve mindful states (defined and discussed later) in consistent manner. As discussed later, such changes derived from the meditation practice on its own and in combination with pharmacological agents, such as allosteric modulators of the GluN2A-selective N-methyl-d-aspartate (NMDA) receptors (which have been shown to restore the gamma oscillations), may have therapeutic benefits in many psychiatric disorders (1, 5).
PV Interneurons and their Neuroanatomical Distribution
Parvalbumin (PV) is a calcium-binding protein involved in calcium signaling and thus plays an important role in the second messenger system and signal transduction in excitable tissues such as muscles and neurons. PV protein has a preferential expression in important neurons in the brain, such as chandelier cells and basket cells; these two neuronal types are otherwise called the PV-positive interneurons, or PV interneurons. They are fast-spiking neurons found in many important brain areas such as the cortex (layers IV and V) and striatum (Figure 1). Embryologically, PV interneurons originate from stem cells in the ventral telencephalon (medial ganglionic eminence); developmentally, they migrate into the cerebral cortex tangentially (6). Because of their characteristic properties (mentioned later), PV interneurons serve as fine-tuning devices for principal neurons (such as pyramidal neurons) by providing them with somatic and perisomatic inhibition.
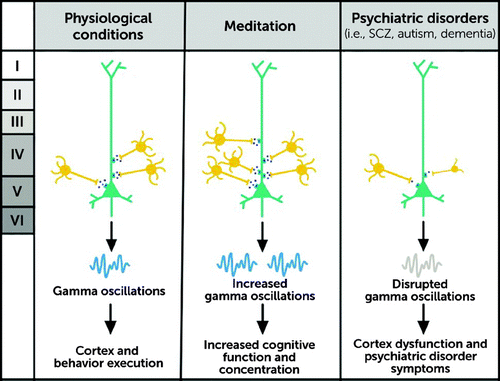
FIGURE 1. Fast-spiking neurons found in important brain areasa
a Under normal physiological conditions, GABA-ergic parvalbumin (PV) interneurons in cortical layers IV and V inhibit pyramidal neurons in cortical layer V by means of GABA receptors. PV interneurons maintain a balance between excitation and inhibition (E–I), resulting in gamma oscillations and spike timing regulation. This allows for proper execution of the cortex, attention, memory, cognitive tasks, and behavior. Meditation practice can lead to changes in brain activity and consequently, brain capacity. Meditating regularly for many years and practicing certain types of meditation, such as mindfulness, verbalization, or visualization meditation, were shown to cause an increase in gamma frequency oscillation activity in various areas of the cortex. Results of meditation included greater focused attention, mindfulness, cognitive function, and concentration. Some psychiatric disorders can be marked by changes in the level of effect that PV interneurons have on pyramidal neurons. In patients with schizophrenia, proteins involved in PV function, PV soma size, and the PV dendritic system are all diminished. Analyses of dementia with Lewy bodies and autism spectrum disorder show a decrease in the number of PV interneurons in the prefrontal cortex. The loss of PV interneuron function in these psychiatric diseases leads to improper maintenance of the E-I balance; gamma frequency oscillation disruption; and, ultimately, the cognitive deficits and symptoms that characterize such disorders. SCZ=schizophrenia.
All PV interneurons are GABA-ergic and categorized into two main types: chandelier cells (most predominant type) and basket cells (second most predominant type). These two types of PV interneurons show fast-spiking action potential firing patterns and cover all nearby pyramidal cells by providing a densely homogenous matrix around them. These special features give them an unparalleled ability to regulate the activity of the nearby pyramidal neurons by perisomatic inhibition (7). The chandelier cells are bipolar neurons with axo-axonic synapses, whereas the basket cells are multipolar neurons (8, 9). The chandelier cells, being axo-axonic are more target specific and synapse only selectively onto the initial axon segments of the receiving pyramidal neurons, whereas basket cells innervate the soma and proximal dendrites of the pyramidal neurons (10, 11). Thus, chandelier cells are the major contributor to the perisomatic inhibition (rather than dendritic inhibition) of the pyramidal neurons, whereas basket cells are the second major contributor. PV interneurons synapse primarily onto the α1-gamma amino butyric acid (GABA) receptors that are present at high levels on the cell body of the pyramidal cells (12). PV interneurons, because of their GABA-ergic actions, can convert the excitatory signals in the pyramidal neurons into inhibitory signals by controlling the action potential generation and timing (13). Thus, they can modulate the incoming excitation from afferent structures to prevent runaway feedforward excitation of the pyramidal cells (14). For the neuronal information processes to occur effectively, the levels of inhibition and excitation must remain in appropriate balance (15). All PV interneurons are GABA-ergic and thus, inhibitory: they are densely present in the prefrontal cortex (PFC; mostly dorsolateral) and the striatum. Of note, the cerebral cortex consists of approximately 20% GABA-ergic interneurons and the other 80% are not GABA-ergic (15). The PV interneurons contribute to about 40% of the cortical GABA-ergic neurons. PV interneurons are also present in the thalamus (reticular nucleus), the cerebellum (Purkinje cells), and the hippocampus. In these locations, their primary function is to regulate the neuronal excitation-firing of the principal-pyramidal cells (16).
Fast-Spiking PV Interneurons and Voltage-Dependent Potassium Channels
Growing evidence supports a fundamental role of the fast-spiking PV interneurons in regulating pyramidal neuron activity to drive appropriate behavioral responses (17). PV interneurons have some special characteristics that facilitate their sustained high-frequency firing and thus make them ideal modulators of the pyramidal neurons (18). PV interneurons fire and repolarize faster, as PV protein rapidly sequesters Ca2+ and attenuates the Ca2+-activated potassium conductance responsible for postspike hyperpolarization (19). This property makes them invariably show a fast-spiking action potential firing pattern. They have low threshold to generate a spike, they are able to fire rapidly without relative fatigue, and they have narrow spikes because of the presence of voltage-dependent potassium channels (KV 3.1 and KV 3.2 types) in them in large density. The KV 3.1 subunits are specifically found in the fast-spiking superficial cortical PV interneurons, whereas the KV 3.2 subunits are specifically found in the fast-spiking deep cortical PV interneurons. The greatest sources of excitation to the cortical inhibitory neurons are the thalamo-cortical glutamatergic neurons that arise mainly from the ventral posteromedial and the posteromedial nucleus of the thalamus. The other major excitatory input to the cortex comes from the amygdala. In the motor cortex, PV interneurons may act as an “inhibitory gate” to achieve an appropriately timed inhibitory response (20).
PV Interneurons and Medium Spiny Neurons (MSNs)
As mentioned earlier, PV interneurons are concentrated in the dorsal striatum, a brain region that mediates motor skills, learning, and conditioned responses to reward-associated stimuli. Similar to the PV interneurons, the MSNs are GABA-ergic (inhibitory) projection neurons and constitute more than 75% of the striatal neurons in humans. The MSNs receive the cortico-striatal glutamatergic afferents mostly from cortical layer-V pyramidal neurons. The striatal PV interneurons give inhibitory inputs to the MSNs and thus downregulate MSN activity through monosynaptic inhibitory signals. In this way, interaction between PV interneurons and MSNs modulates the striatal output and enhances the performance during associative learning. Optogenetic studies in animal models show that the influence of the PV interneurons on the MSNs is selective to only the initial stages of learning and reward conditioning and gradually gets attenuated as learning goes on (i.e., this influence diminishes with training and experience) (21). The dorsal striatum integrates the information received from the cortical and thalamic neurons about the various sensory, motivational, and motor states and thus facilitates the selection of actions that can achieve the best outcomes. The mesenecephalic dopaminergic neurons innervating the striatum provide a means by which experience shapes the strength of the corticostriatal synapses of the MSNs (synaptic potentiation and neuroplasticity) (22). Depending on their dopamine receptor expression, their axonal projection (striatoniagral versus striatopallidal), and the resultant action on the thalamus, MSNs are subdivided into two major subpopulations of approximately equal number: striatonigral direct pathway MSNs (dMSNs) and striatopallidal indirect pathway MSNs (iMSNs) (23). The striatonigral dMSNs robustly express D1 receptors, and their action is excitatory to the thalamus, whereas the striatopallidal iMSNs robustly express D2 receptors, and their action is inhibitory to the thalamus.
In this context, it is important to mention the defining characteristics of GABA-ergic interneurons. The major defining factor for GABA-ergic interneurons is their ramification pattern (as seen in the chandelier and basket cells) and their accompanying subcellular target specificity. These features contribute to their collateral inhibition actions on the principal or pyramidal neurons. The microcircuit architecture of the PV interneurons demonstrates connections at nearly every local pyramidal neuron, and these interneurons are involved in neocortical inhibition in general.
Complex Role of PV interneurons in Operations of Brain Microcircuits
Because of their special characteristics, PV interneurons are involved in both the feedforward and feedback inhibition of the neuronal circuits that causes modulation/enhancement of signal-to-noise ratios in these circuits. Thus, PV interneurons produce complex influences on brain functioning because of their important regulatory roles over the principal neurons. PV chandelier cells may play a dual role in the cortical neurocircuits, helping to activate quiescent pyramidal neurons while inhibiting active ones in a concerted and meaningful manner (24). PV basket cells play an important role in thalamus-generated feedforward inhibition of long-range cortical input and subcortical neuro-modulatory input (9). PV interneurons have other functions as well, such as complex microcircuit operations including pattern separation, gain modulation of sensory responses, and phase precession (25). Phase precession is a complex memory (encoding) mechanism seen in rodent hippocampal place cells (26). In this mechanism, the binding and compressing of sequential events occur by means of which the information gets coded in the memory engram (memory trace).
To have a successful memory of the sequential experiences through associative learning in the brain, timing of neuronal spiking is critical. Mechanistically, this process requires the association of sequential events by binding and compressing mechanisms that, in turn, are dependent on a coordinated relationship between neuronal network oscillations and single-cell spiking. During phase precession, active hippocampal neurons (place cells) rhythmically spike in coordination with the local theta-frequency oscillation (5–10 Hz). This process is called “precession” because these place cells spike slightly faster than the theta oscillation as the rodent runs through specific locations, and it results in sequences of locations being encoded at different phases of theta oscillations. Because of this mechanism, the hippocampus works much like a global positioning system and a chronobiographer with regard to the encoding of spatial and temporal memories.
PV Interneurons Modulate the Gamma EEG Oscillations (Gamma Band)
The function of the PV interneurons is more complex than just inhibition of pyramidal neurons in the cortex. They generate feedback and feedforward inhibition of cortical neurons, and they are critically involved in the generation of neuronal network oscillation that can be detected in the EEG in the form of oscillations or bands (beta, alpha, theta, delta, and gamma, in order of increasing frequency). These oscillations or bands, generated by various brain regions, are associated with certain brain neurophysiological processes and are linked to perceptions, thought processes, and cognitive functions, all of which, in turn, influence human emotions and behavior. For example, beta (especially high-beta) signifies the expectancy of stimulus-reward and anxiety, and low-beta dominates our normal waking state (state of active alertness, external attention) of consciousness. The alpha band is associated with quiet alertness (internal attention), and theta signifies emotional processing and mood regulation. The delta band reflects deep sleep and meditative states, and gamma is associated with sensory integration and sensory processing (27).
The fast-spiking PV interneurons regulate the activity of neural networks through GABA-ergic inhibition of local excitatory neurons. Although many classes of GABA-ergic neurons are active during gamma oscillations, the PV interneurons show the strongest coupling to gamma activity (28). Synchronous activity of the fast-spiking PV interneurons generates gamma oscillations (30–80 Hz) (29). PV interneurons are especially capable of supporting gamma oscillations because of their aforementioned special characteristics. The kinetics of gamma oscillations vary depending on the structure of the brain under study and are explained by the inhibitory-inhibitory model or the E-I model. PV interneurons play a critical role in the E-I model of gamma oscillations. It is also known that, in neuronal networks, patches of gamma oscillations can interact with one another (intrafrequency coupling). This coupling phenomenon between gamma oscillations is an electrophysiological measure of local and long-distance neuronal communication and can be phase-phase, phase-amplitude, or amplitude-amplitude coupling.
Modulation of gamma oscillations by theta, delta, slow, spindle, and ultraslow oscillations has been demonstrated (30). Normal gamma oscillations are correlated with the performance of a variety of cognitive tasks, including the allocation of attention and working memory (31). Gamma oscillations mediate long-term neuronal plasticity as well. Precise, fast inhibition provided by PV-positive neurons along with slow inhibition ascribed to cholecystokinin (CCK)-positive interneurons both modulate plasticity. PV- and CCK-positive interneurons have been shown to constitute and enable a network state that promotes the formation of long-term synaptic/neuronal plasticity (32).
Yoga, Meditation, and Mindfulness
As proposed originally in ancient India, yoga is inclusive of meditation, and meditation is inclusive of mindfulness. The sixth and seventh limbs of the all-encompassing eight-limbed yoga are based in meditation (33). Mindfulness (or, in Pali, satipatthana) is one among the two types of meditation (the other type is called concentration or stable attention; samatha, in Pali). Meditation, simply stated as “training of the mind,” has been recognized as a spiritual and healing practice in many parts of the world and in many cultural and belief systems for thousands of years. Although it is beyond the scope of this article to discuss the exact timeline and different forms of meditative practices and how they emerged historically and philosophically, this is a fascinating and rich field of study (34). Meditation is the conscious and purposeful self-regulation of one’s attention and bodily movements to achieve stillness of one’s body. This is supposed to induce nonreactivity of one’s mind so that it becomes possible to observe nonjudgmentally the ongoing stream of experiences as they arise when one tries to establish focused and flexible attention on the breath or any meditation object during the act of meditation or contemplation (33). Accordingly, meditation essentially involves at least three components: attentional control, affect regulation, and heightened self-awareness or heightened perception or altered consciousness that collectively support the physical and mental health, resilience, well-being, and behavior change (35).
Lutz et al. (36) proposed a theoretical framework for meditative practice by grouping it into two broad categories: focused attention meditation and open-monitoring meditation. Zen (found in the historical tradition of Mahayana Buddhism and Taoism), Vipassana (found in the historical traditions of Theravada Buddhism), and Tibetan Buddhist meditation traditions (Vajrayana) have a core practice of focused attention and open monitoring. Focused attention is a popular style of Buddhist practice, consisting of sustaining selective attention (exclusive attention) moment by moment on a chosen meditation object (kasina, in Pali), commonly the breath. Alternatively, in open-monitoring meditation, the practitioner aims to remain only in the monitoring state, attentive moment by moment to anything that occurs in the practitioner’s experience without focusing on any explicit object; thus, this type of attention is known as inclusive attention (36).
There is a third type of meditation, transcendental meditation, in which the meditator transcends their own thoughts and activity through the silent chant of a mantra (e.g., a monosyllabic sound). Although its methodology is close to concentration practice, its essential marker is the absence of focused attention and open monitoring during the meditative practice (37). This third category of meditation might account for the genesis of the belief that the brain has a greater capacity to transcend the boundaries of logic and reason and thereby can experience a heightened state of awareness (38). Interestingly, many Eastern thinkers believe that there are additional and further heightened states of awareness such as the “thoughtless awareness state” (turiya in Sanskrit), defined by some as a fourth state of consciousness (the other three states being sleeping, waking, and dreaming) (38).
Meditation has its roots in many diverse ancient contemplative traditions that mutually share common themes and principles (39). Mindfulness refers to an alert and open mode of perceiving and monitoring all the mental contents from moment to moment, including sensations, thoughts, and emotions. In essence, mindfulness involves both open monitoring and focused attention (36). In general, mindfulness practice is initiated with a period of focused attention and gradually transitions to open monitoring (40). Mindfulness practice allows any thoughts, feelings, or sensations just to arise and pass away in one’s awareness while the individual maintains a specific attentional stance: thus, eventually leading to a state of detached observation of these mental contents. One concise and helpful formulation states that mindfulness is the awareness that arises through purposeful, nonjudgmental attentiveness to present-moment experience (41). The fundamental feature is persistent, repeated engagement with an attention set that is considered as open, nonjudgmental awareness and observation of ongoing mental processes (40). Although meditation has been practiced in quite diverse forms and in different parts of the world, virtually all religions or cultures have common attributes of mind training that share characteristics with, or are similar to, principles of meditation practice. Later in chronological development, many of the modern Western forms of meditation techniques or practices were designed to ameliorate so-called pathological states of the mind; for instance, the states of heightened anxiety or cognitive decline (39).
Meditation in the Context of Neurobiology and EEG Bands
Studies of Tibetan Buddhist meditators using different meditation techniques support that each kind of meditation leads to distinct mind-brain states (42). A brain state can be defined as a reliable pattern of activity or connectivity (or both) in multiple large-scale brain networks (43, 44). Meditation involves training of the mind and brain to obtain the meditative states that can have accompanying measures of change such as mood or behavior or brain activity while the practitioners are established in such states. These studies can elucidate how these states influence the brain, mood and behavior (45–47). A recent review summarized what is known about characterizations of brain regions involved in the three core components of mindfulness meditation: attentional control (external attention) is mainly grounded in the anterior cingulate cortex (ACC) and the striatum; emotional regulation is grounded in the prefrontal regions, limbic areas and the striatum; and self-awareness (internal attention) mainly arises from the insula, medial PFC, and posterior cingulate cortex and precuneus brain regions (the default mode network) (35).
Neural oscillations detected by EEG in the form of the five bands reflect synchronization of neural activity triggered by a simple sensory event or a complex cognitive process. These oscillations are part of the complex communication by large groups of neurons to serve the general brain functions. To better clarify and understand the patterns of EEG oscillations in mindfulness meditation, a systematic review examining 56 studies with a total of 1,715 subjects revealed that mindfulness meditation is most commonly associated with enhanced alpha and theta power, compared with an eyes-closed resting state when an explicit task is not being performed. These authors also reported that there are no consistent patterns associated with beta, delta, and gamma bandwidths during mindfulness meditation (48). Nevertheless, a few studies have found that, in contrast to a focused attention task, the thoughtless emptiness task showed significant central and parietal gamma decreases, suggesting that meditative conscious awareness might be different from the mentally focused states (49). This might be attributable to changes in self-referential and attentional networks and may be related to momentary sensory awareness or to the conscious representation of mental content (50). The occipital gamma increase is most significant in experienced meditators who had daily meditative practice of more than 10 years with enhanced sensory awareness (50). Creative thinking involves greater capacity for attentional tasks associated with widespread enhancement of power and coherence of beta and frontal increase in theta power (51). An interesting hypothesis is that the meditative state is a function of brain capacity or even encompasses an inherent ability for neuroplasticity. This neuroplasticity is recruited by neural oscillations through synchronization of alpha, theta, and gamma waves (52). A study examined the EEG changes related to Acem meditation, a secular form of meditation involving sound repetition, and showed significantly increased theta power, which was higher in the frontal and temporal-central regions, as compared with alpha power, which was significantly higher in posterior (specifically the occipital) regions (53).
A quantitative analysis of Zen meditation in 20 healthy adults found that, during Zen meditation, there is an increase in frontal fast theta power, which reflects enhanced mindful awareness. Also, there was increase in the alpha power, which reflects enhanced internalized attention. The results suggest that internalized attention and mindfulness contribute to different permutations of psychophysiological properties (54). Primate modeling that investigated visual stimuli sheds some light on the potential complexity of alpha- and theta-band interrelationships here; investigators found that, in healthy adult monkeys (e.g., Macaca mulatta), primary visual cortex gamma activity is generated in the superficial and granular layers and is associated with the processing of visual input and alpha-band activity in deeper layers that is associated with arousal and cognition. There is coupling between the alpha phase in the deeper layers and gamma amplitude in granular and superficial layers (55). However, at present, the precise interrelationship between these different oscillations, and its significance, have not yet been elucidated.
Gamma-frequency oscillations originate from PV interneurons (GABA-ergic neurons), and GABA has been implied in meditative states (56, 57); therefore, in the context of meditation, gamma frequency oscillations deserve special mention. The gamma oscillation of frequency is reflective of heightened concentration, sensory awareness and high levels of cognitive functioning. Studies examining meditation visualization and verbalization techniques showed that gamma-frequency power increased in the right posterior occipital lobe during visualization meditation (in which the focus is placed on an imagined image) and in left central-temporal regions during verbalization meditation (e.g., mantra chanting) (4, 40). Synchrony is a newer method of measuring varying EEG signals, and it has been shown that gamma synchronization acts as a distributed unifying mechanism in human cognitive activity (58). EEG recordings carried out in 11 meditators correlated with the gamma power (30–250 Hz), particularly the high-frequency gamma range (100–245 Hz), during meditation. Improved and higher levels of mindfulness were related to increased high-frequency gamma in the cingulate and somatosensory cortices (59). An interesting observation from a study using low-resolution electromagnetic tomography analysis functional imaging of EEG gamma (35–44 Hz)-frequency band activity found that gamma activity differed significantly between different types of meditation (4).
PV Interneurons in Emotions, Cognition, and Behavior: Implications for Neuropsychiatry
Impact of Brain Functions Associated With PV Neurons and Meditation
The fascinating capacity of the brain lies within its complex structure and its potential for the formation of new synaptic junctions, plasticity, and complex synaptic transmission. Of primary structural significance in the brain is the PFC, which is involved in executive functions, attention, and working memory. Overall, complex networks in the PFC balance the experiences with the perceptions in order to determine the appropriate actions. Many psychiatric conditions directly or indirectly relate to the impairments in PFC functioning. In 2003, intracranial recordings made from two patients with epilepsy showed the first evidence of gamma oscillations that were determined to be widely responsible for perceptual processes and contributory to the maintenance of multiple aspects of cognition and information processing, including working memory, across species (60). Working memory information in neuronal spiking was linked to brief, narrow-band gamma bursts, because these bursts increased during encoding and decoding, and with working memory load (i.e., the amount of information that must be held in memory at any given time to accomplish a given task) (60, 61). The gamma-band response was larger during selective attention (62) and also influences associative learning in humans (63).
The PV interneurons are the major source of gamma oscillations and maintain a high level of inhibition through the mediodorsal thalamic inputs relative to excitation in the pyramidal neurons (17, 64). The roles of the thalamus (the main subcortical sensory relay station) and the hippocampus in associative learning is established, and it is interesting to note that these two brain areas are also related to the sources of gamma oscillations. PV-neuron-generated gamma oscillations mediate cognitive, perceptual, and behavioral processes (65). By balancing the networks of excitatory-inhibitory (E-I) neurons and managing the multimodal input from various brain regions for cortical control, the PFC ultimately initiates and guides behavior (13).
Role of PV interneurons in Neuropsychiatric Disorders
A recent review by Ruden et al. (1) highlights the role of PV neurons in many neuropsychiatric disorders, including schizophrenia, Alzheimer’s disease, autism spectrum disorder (ASD), bipolar disorder, and epilepsy. The functions of the fast-spiking PV interneurons are heavily diminished in the PFC in numerous psychiatric disorders, including schizophrenia and ASD (64). PV interneurons are powerful regulators of E-I balance in the PFC, and they help optimize the representation and processing of supramodal (i.e., independent of the sensory modality) information in the PFC. The voltage-dependent potassium channels (KV 3.1 and KV 3.2 types) which are located in the PV interneurons are reduced in number in untreated schizophrenia (66) and presumably in ASD as well (67). In dementia with Lewy bodies (DLB), an alpha-synucleinopathy, there is partial loss of the number of the PV interneurons due to long-term calcium overload and impaired mitochondrial functions, thus leading to deficits in the gamma-frequency activity (68). The cognitive deficits in DLB due to dysfunction of dorsolateral PFC (DLPFC) networks are linked to disturbances in gamma-frequency oscillations (generated in the layer III of the DLPFC). Although the density of the cortical PV interneurons has not been observed to be altered in schizophrenia, the levels of expression of several proteins involved in the regulation of the functions of the PV interneurons, as detected in their transcriptome expression patterns, have been found to be lower in this severe mental illness (69).
Because the PV interneurons are powerful regulators of the E-I activity in the PFC, reductions of their number or functioning can cause deficits in the E-I balance that alter the gamma-frequency oscillations. Such deficits are associated with anhedonia, social withdrawal, deficits in working memory, and diminished cognitive flexibility and control, which are seen in many psychiatric disorders, including schizophrenia, ASD, and major depressive disorder (17, 70, 71). In schizophrenia, the PV interneuron inhibition onto the pyramidal cells is decreased (impaired gamma oscillations) which has been hypothesized to be a key locus of the pathophysiology underlying schizophrenia (72, 73). The PV interneurons show marked decreases in size in both the cell body and the dendritic system in individuals with schizophrenia (74). In a postmortem study that compared the hippocampal specimens of bipolar affective disorder patients with those from healthy control subjects, showed reduced volume of the nonpyramidal cell layers, reduced cell body volume, and reduced messenger RNA (mRNA) levels in PV interneurons in the bipolar disorder group (75). Depression is associated with somatostatin-positive GABA-ergic interneurons in the DLPFC, whereas decreased PV interneuron mRNA levels appears to distinguish bipolar disorder from major depressive disorder (76). From a therapeutic (disorder modifying) perspective, the GABA neurons, when exposed to serotonin (5-HT1A) agonists and the neurotrophic agent T-817MA, have been proposed to prevent the onset or progression (or both) of schizophrenia (77). As mentioned above, PV interneurons maintain a high level of inhibition through the mediodorsal thalamic inputs relative to excitation in pyramidal neurons (64). By regulating spike timing and oscillatory patterns, they contribute to proper execution of PFC networks and, in turn, behaviors. It is speculated that either cell-autonomous factors or altered inputs can lead to multiple gene (presumably) pathologic expression patterns in PV interneurons that contribute to cognitive deficits in several psychiatric disorders, including schizophrenia (78).
A study conducted in 12 patients with schizophrenia, compared with 12 healthy control subjects, found that neural circuit impairments resulted from failure of gamma-band synchronization (79). In the first report of distinctive patterns of gamma activity in three symptom syndromes seen in patients with schizophrenia (defined as psychomotor poverty, disorganization, and reality distortion), 35 patients with schizophrenia were compared with 35 age- and gender-matched healthy control subjects, and the sample with schizophrenia showed generally reduced gamma activity. Analysis of the responses to 40 target and 40 nontarget auditory stimuli showed that reality distortion was correlated with increased gamma, whereas psychomotor poverty and disorganization were correlated with decreased gamma synchronicity, and the authors suggested that this may explain disturbances in integrated brain function characteristic of schizophrenia and provide complementary insights to the established slower frequency event-related potential dysfunctions in schizophrenia (80). A 2012 review by Gandal et al. (81) hypothesized that GABA-ergic circuit deficits—particularly the disruption of fast-spiking PV-expressing interneurons—result in pathophysiological deficits that are linked to core symptoms of schizophrenia. Furthermore, dysfunctional fast-spiking interneuron activity appears to be a final common pathway in schizophrenia that could explain gamma abnormalities in cortical inhibitory pathways. The review also speculates that gamma abnormalities are a biomarker or endophenotype for treatment-resistant symptoms of schizophrenia—most notably, negative symptoms—and might be used ultimately as a rational target for therapeutic intervention (81). High prevalence of reductions in PV interneurons or reductions in GAD67 (GABA-ergic enzyme protein) expression in the mPFC are observed with decreases in task-evoked gamma oscillations, which is a recognized feature in schizophrenia. In schizophrenia, environmental factors, too, such as social isolation, are posited by some as pathoetiological factors that converge with genetic causative factors (multifactorial etiology). This could be linked to PV interneuron susceptibility to oxidative stress from these same environmental stressors (e.g., social isolation) because of downregulation of peroxisome proliferator-activated receptor-γ coactivator-1α, a transcription coactivator that is essential in cellular energy metabolism and has antioxidant properties (82, 83).
Dysregulation of the GABA-ergic signaling has been implicated in the etiology of ASD, anxiety disorders, and epilepsy (17). For example, a postmortem analysis of neocortical tissue from 11 patients with ASD and 10 matched control subjects that examined PFC PV interneurons detected significant reductions in the autistic patients compared with control subjects. This dysregulation of GABA-ergic signaling is posited to affect the balance of E-I and alter gamma-wave oscillations in the cerebral cortex of autistic subjects (71). In some brain regions, animal studies have shown that the inhibition of PV interneurons reduces anxiety and improves cognitive performance. For example, in a mouse model, rhythmic brain stimulation of the ACC and inhibition of PV interneurons can lead to a reduction in anxiety and the formation of new myelin-producing oligodendrocytes (84). Also, in a murine model, stimulation of PV interneurons in the dentate gyrus produced an anxiolytic effect, with an increase in learning and memory, and enhanced the extinction of fear-causing memories (85).
Current research posits that the PV interneurons are implicated in many psychiatric disorders (86). Stress can inactivate PV interneurons and accelerates the loss of their spiny dendritic processes. This might disrupt global neuronal excitation or inhibition in the primary somatosensory cortex and result in perceptual disturbance. Interestingly, pharmacogenetic and behavioral manipulations that enhance PV interneuron activity can prevent stress-induced dendritic spine loss and overexcitation (87). Deep brain stimulation of thalamocortical neurons, for example, increased the activity of PV interneurons in brain cortex (88). Disturbances in NMDA receptor (NMDAR) signaling in fast-spiking PV interneurons can induce cognitive deficits as well as psychosis-like symptoms in humans (29). The newly investigated psychotropic with antidepressant effects, ketamine, produces at least some of its antidepressant action by means of inhibiting NMDARs expressed on GABA-ergic interneurons, leading to pyramidal cell disinhibition and an enhancement of excitatory glutamatergic neurotransmission in the medial PFC (mPFC) and, potentially, other mood-relevant corticolimbic brain pathways (89, 90). Ketamine, notably at antidepressant doses, prevented stress-induced dendritic spine loss, increased PV interneuron activity, prevented net loss of PV axonal boutons, and modulated dendritic spine plasticity in response to stress (91).
The cortical silent period (CSP), a measure related to transcranial magnetic stimulation (TMS) in meditators, found that there is a significant increase in the CSP linked to GABA-ergic cortical inhibition, a mechanism implicated in improved cognitive performance and enhanced emotional regulation (92). Meditation-related increases in GABA-B seems to modulate cortical inhibition. A study of 40 undergraduate meditators demonstrated that 20-minute daily meditation sessions reduced fatigue, increased vigor, and produced a marked increase in stress-related cortisol and an increase in immunoreactivity. These meditation sessions also reduced anxiety, improved attention and cognition, and increased rhythmic electrical activity in brain areas related to emotional control (93). Optogenetic technology in a mouse model found that inhibiting PV interneurons suppressed gamma oscillations and, conversely, that stimulating PV interneurons generated gamma-frequency rhythmicity (94). However, despite promising lines of inquiry using even powerful new genetic engineering techniques, it remains unknown if these deficits in gamma synchronicity in humans can be clinically and therapeutically corrected so as to demonstrate meaningful clinical improvements. These pieces of evidence all converge to suggest that PV interneurons are involved in basic but essential functions that help regulate somatic and mental states. In essence, and a promising finding, meditation and mindfulness techniques produce neurobiological changes in the brain and physiologic improvements in body function that have been shown to be enduring for patients who continue to practice these techniques (39). More human studies are necessary to ascertain the exact roles of PV interneurons and gamma oscillations in these states and various psychiatric disorders.
Discussion
Neural oscillations are generated when there is sensory stimulation or event-related tasks as manifested by synchronization of neural (network) activity. These oscillatory networks are necessary for general brain functions, including communication and associations, and also memory retrieval. The oscillatory systems in specific frequencies (delta, theta, alpha, and gamma) act as resonant communication networks through large populations of neurons (95). More specifically, gamma-band synchronization, which can emerge in any network of an excitatory or inhibitory group of neurons, plays a role in multiple cognitive functions. Shunting inhibition rather than hyperpolarization may provide increased robustness to gamma oscillations (96). For effective communication within cortical areas during cognitive processing in humans, a transient coupling between low-frequency theta bands (4–8 Hz) and high-frequency gamma bands (80–150 Hz) is required, so the theta phase has a modulatory influence on gamma amplitude. There is speculation that alpha rhythms also modulate the strength of gamma-band synchronization and that alpha rhythms are strong, particularly over the posterior parietal cortex, a region involved in stimulus selection (97). However, the precise interrelationship between these different oscillations remains unclear.
An added layer of complexity arises when these states are mediated or modulated by mindfulness or meditative states, and these states have important implications for therapeutic interventions and wellness in all their diverse aspects. As with alpha and theta bands, both in open monitoring and focused attention states, there is higher parieto-occipital (60–110 Hz) gamma amplitude than in control subjects as a trait effect observed during meditation, and it is directly proportional to the participant’s duration of meditation experience (interestingly, the investigators were also able to rule out confounding gamma activity that had originated as eye or muscle activity artifacts) (98). In summary, PV interneuron-generated gamma oscillation may function in conscious perception (99), memory-based cognitive functions (100), and attentive processing of information (101).
Network oscillations are known to mediate internal representations of neural information, the flow of information in neural circuits, and storage and retrieval of information in neural circuits. There is a consistent finding across many studies that meditation can beneficially modulate alpha and theta oscillations and, to some extent, gamma oscillations as well. Studies have also found that PV interneuron-generated gamma oscillations mediate cognitive, perceptual, and behavioral processes (65). Specifically, long-term meditation has been shown to produce changes in brain oscillations of specific frequencies, including alpha, theta, and gamma bands. This suggests that meditation might be an effective treatment modality to create long-lasting therapeutic effects and recovery from psychiatric disorders through approaches involving modulations of network oscillations. Deficits in gamma oscillations, for example, may be reflective of aberrant network functioning and might be a potential biomarker leading to therapeutic inroads into the treatment of specific psychiatric disorders (81). More research is warranted to understand the brain oscillations and the neurophysiological processes resulting from the groups of neurons that generate them during meditation, which, in turn, might have clinical applications, both diagnostic and therapeutic.
1 : Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology 2021; 46:279–287Crossref, Medline, Google Scholar
2 : Mechanisms of gamma oscillations. Annu Rev Neurosci 2012; 35:203–225Crossref, Medline, Google Scholar
3 : Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol 1980; 50:19–24Crossref, Medline, Google Scholar
4 : Brain sources of EEG gamma frequency during volitionally meditation-induced, altered states of consciousness, and experience of the self. Psychiatry Res 2001; 108:111–121Crossref, Medline, Google Scholar
5 : Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. Neuron 2016; 89:983–999Crossref, Medline, Google Scholar
6 : Development and functional diversification of cortical interneurons. Neuron 2018; 100:294–313Crossref, Medline, Google Scholar
7 : Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 2011; 31:13260–13271Crossref, Medline, Google Scholar
8 : Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 2004; 5:793–807Crossref, Medline, Google Scholar
9 :
10 : GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 1997; 7:476–486Crossref, Medline, Google Scholar
11 : Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci 2007; 27:1139–1150Crossref, Medline, Google Scholar
12 : Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA 1996; 93:11939–11944Crossref, Medline, Google Scholar
13 : Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 2001; 293:1159–1163Crossref, Medline, Google Scholar
14 : Cellular mechanisms of epilepsy: a status report. Science 1987; 237:157–164Crossref, Medline, Google Scholar
15 : Activation of type-1 cannabinoid receptor shifts the balance between excitation and inhibition towards excitation in layer II/III pyramidal neurons of the rat prelimbic cortex. Pflugers Arch 2015; 467:1551–1564Crossref, Medline, Google Scholar
16 : Parvalbumin-containing GABAergic interneurons in the rat neostriatum. J Comp Neurol 1990; 302:197–205Crossref, Medline, Google Scholar
17 : PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits 2018; 12:37Crossref, Medline, Google Scholar
18 : K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci 1999; 19:9332–9345Crossref, Medline, Google Scholar
19 : Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA 2000; 97:13372–13377Crossref, Medline, Google Scholar
20 : Parvalbumin-expressing GABAergic neurons in primary motor cortex signal reaching. Cell Rep 2017; 20:308–318Crossref, Medline, Google Scholar
21 : Parvalbumin interneurons modulate striatal output and enhance performance during associative learning. Neuron 2017; 93:1451–1463.e4Crossref, Medline, Google Scholar
22 : The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res 2010; 183:148–167Crossref, Google Scholar
23 : D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990; 250:1429–1432Crossref, Medline, Google Scholar
24 : State-dependent function of neocortical chandelier cells. J Neurosci 2011; 31:17872–17886Crossref, Medline, Google Scholar
25 : Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 2014; 345:1255263Crossref, Medline, Google Scholar
26 : Phase precession: a neural code underlying episodic memory? Curr Opin Neurobiol 2017; 43:130–138Crossref, Medline, Google Scholar
27 : Depression biomarkers using non-invasive EEG: a review. Neurosci Biobehav Rev 2019; 105:83–93Crossref, Medline, Google Scholar
28 : Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 2007; 27(31):8184–8189Crossref, Medline, Google Scholar
29 : A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 2012; 17:537–548Crossref, Medline, Google Scholar
30 : Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 2012; 14(4):345–367Crossref, Medline, Google Scholar
31 : The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 2008; 34:927–943Crossref, Medline, Google Scholar
32 : Cell-specific synaptic plasticity induced by network oscillations. eLife 2016; 5:e14912Crossref, Medline, Google Scholar
33 : Yoga and Mindfulness Based Cognitive Therapy: A Clinical Guide. Cham, Switzerland, Springer, 2014Google Scholar
34 : A systematic review of transcendent states across meditation and contemplative traditions. Explore (NY) 2018; 14:19–35Crossref, Medline, Google Scholar
35 : The neuroscience of mindfulness meditation. Nat Rev Neurosci 2015; 16:213–225Crossref, Medline, Google Scholar
36 : Attention regulation and monitoring in meditation. Trends Cogn Sci 2008; 12:163–169Crossref, Medline, Google Scholar
37 : Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn 2010; 19:1110–1118Crossref, Medline, Google Scholar
38 : The fourth state of consciousness: the Thuriya Avastha. Psychiatry Clin Neurosci 1995; 49:107–110Crossref, Medline, Google Scholar
39 : Meditation and mindfulness in clinical practice. Child Adolesc Psychiatr Clin N Am 2014; 23:487–534Crossref, Medline, Google Scholar
40 : Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull 2006; 132:180–211Crossref, Medline, Google Scholar
41 : Mindfulness-based interventions in context: past, present, and future. Clin Psychol Sci Pract 2003; 10:144–156Crossref, Google Scholar
42 : Meditation alters perceptual rivalry in Tibetan Buddhist monks. Curr Biol 2005; 15:R412–R413Crossref, Medline, Google Scholar
43 : Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012; 59:750–760Crossref, Medline, Google Scholar
44 : Training brain networks and states. Trends Cogn Sci 2014; 18:345–350Crossref, Medline, Google Scholar
45 : Molecular mechanisms of meditation. Mol Neurobiol 2013; 48(3):808–811Crossref, Medline, Google Scholar
46 : Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett 2007; 421:16–21Crossref, Medline, Google Scholar
47 : Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. J Neurosci 2012; 32:5242–5249Crossref, Medline, Google Scholar
48 : A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci Biobehav Rev 2015; 57:401–410Crossref, Medline, Google Scholar
49 : Decreased electrophysiological activity represents the conscious state of emptiness in meditation. Front Psychol 2014; 5:99Crossref, Medline, Google Scholar
50 : Occipital gamma activation during Vipassana meditation. Cogn Process 2010; 11:39–56Crossref, Medline, Google Scholar
51 : Creativity related cortex activity in the remote associates task. Brain Res Bull 2007; 73:96–102Crossref, Medline, Google Scholar
52 : From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Med Hypotheses 2010; 75:218–224Crossref, Medline, Google Scholar
53 : Increased theta and alpha EEG activity during nondirective meditation. J Altern Complement Med 2009; 15:1187–1192Crossref, Medline, Google Scholar
54 : Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol 2005; 55:199–207Crossref, Medline, Google Scholar
55 : Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol 2012; 22:2313–2318Crossref, Medline, Google Scholar
56 : Effects of yoga versus walking on mood, anxiety, and brain GABA levels: a randomized controlled MRS study. J Altern Complement Med 2010; 16:1145–1152Crossref, Medline, Google Scholar
57 : Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses 2012; 78:571–579Crossref, Medline, Google Scholar
58 : Studying single-trials of phase synchronous activity in the brain. Int J Bifurcat Chaos 2000; 10:2429–2439Crossref, Google Scholar
59 : What it means to be Zen: marked modulations of local and interareal synchronization during open monitoring meditation. Neuroimage 2015; 108:265–273Crossref, Medline, Google Scholar
60 : Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13:1369–1374Crossref, Medline, Google Scholar
61 : Gamma and beta bursts underlie working memory. Neuron 2016; 90:152–164Crossref, Medline, Google Scholar
62 : Selective attention enhances the auditory 40-Hz transient response in humans. Nature 1993; 364:59–60Crossref, Medline, Google Scholar
63 : Coherence of gamma-band EEG activity as a basis for associative learning. Nature 1999; 397:434–436Crossref, Medline, Google Scholar
64 : From hiring to firing: activation of inhibitory neurons and their recruitment in behavior. Front Mol Neurosci 2019; 12:168Crossref, Medline, Google Scholar
65 : Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 2010; 34:981–992Crossref, Medline, Google Scholar
66 : Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Mol Psychiatry 2014; 19:573–579Crossref, Medline, Google Scholar
67 : The parvalbumin hypothesis of autism spectrum disorder. Front Cell Neurosci 2020; 14:577525Crossref, Medline, Google Scholar
68 : Partial loss of parvalbumin-containing hippocampal interneurons in dementia with Lewy bodies. Neuropathology 2011; 31:1–10Crossref, Medline, Google Scholar
69 : Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry 2018; 23:1606–1613Crossref, Medline, Google Scholar
70 : Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 2015; 77:1031–1040Crossref, Medline, Google Scholar
71 : The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cereb Cortex 2017; 27:1931–1943Medline, Google Scholar
72 : Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35:57–67Crossref, Medline, Google Scholar
73 : Impaired GABAergic neurotransmission in schizophrenia underlies impairments in cortical gamma band oscillations. Curr Psychiatry Rep 2013; 15:346Crossref, Medline, Google Scholar
74 : Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport 2002; 13:713–717Crossref, Medline, Google Scholar
75 : Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry 2011; 68:340–350Crossref, Medline, Google Scholar
76 : GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 2011; 14:721–734Crossref, Medline, Google Scholar
77 : New pharmacotherapy targeting cognitive dysfunction of schizophrenia via modulation of GABA neuronal function. Curr Neuropharmacol 2015; 13:793–801Crossref, Medline, Google Scholar
78 : Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 2014; 26:22–26Crossref, Medline, Google Scholar
79 : Abnormal neural synchrony in schizophrenia. J Neurosci 2003; 23:7407–7411Crossref, Medline, Google Scholar
80 : Symptom profile and “gamma” processing in schizophrenia. Cogn Neuropsychiatry 2001; 6:7–19Crossref, Google Scholar
81 : Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012; 62:1504–1518Crossref, Medline, Google Scholar
82 : Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Brain Res Rev 2006; 52:293–304Crossref, Medline, Google Scholar
83 : Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front Behav Neurosci 2013; 7:116Crossref, Medline, Google Scholar
84 : Rhythmic brain stimulation reduces anxiety-related behavior in a mouse model based on meditation training. Proc Natl Acad Sci USA 2017; 114:2532–2537Crossref, Medline, Google Scholar
85 : DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med 2016; 16:91–102Crossref, Medline, Google Scholar
86 : Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13:107–120Crossref, Medline, Google Scholar
87 : Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. Mol Psychiatry 2018; 23:1614–1625Crossref, Medline, Google Scholar
88 : Brain stimulation patterns emulating endogenous thalamocortical input to parvalbumin-expressing interneurons reduce nociception in mice. Brain Stimul 2018; 11:1151–1160Crossref, Medline, Google Scholar
89 : Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17:2921–2927Crossref, Medline, Google Scholar
90 : Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 2018; 23:801–811Crossref, Medline, Google Scholar
91 : Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl Psychiatry 2018; 8:272Crossref, Medline, Google Scholar
92 : Meditation-related increases in GABAB modulated cortical inhibition. Brain Stimul 2013; 6:397–402Crossref, Medline, Google Scholar
93 : Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci USA 2007; 104:17152–17156Crossref, Medline, Google Scholar
94 : Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459:698–702Crossref, Medline, Google Scholar
95 : Brain oscillations in perception and memory. Int J Psychophysiol 2000; 35:95–124Crossref, Medline, Google Scholar
96 : Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 2006; 49:107–117Crossref, Medline, Google Scholar
97 : Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 2009; 32:209–224Crossref, Medline, Google Scholar
98 : Increased gamma brainwave amplitude compared to control in three different meditation traditions. PLoS One 2017; 12:e0170647Crossref, Medline, Google Scholar
99 : Consciousness and the binding problem. Ann N Y Acad Sci 2001; 929:123–146Crossref, Medline, Google Scholar
100 : Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 2004; 8:347–355Crossref, Medline, Google Scholar
101 : Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol Paris 2006; 100:182–193Crossref, Medline, Google Scholar



