Cumulative Effect of Head Injuries on Nonmotor Outcomes in Parkinson’s Disease
Abstract
Objective:
Parkinson’s disease (PD) is a neurodegenerative movement disorder that is a result of dopamine depletion in the basal ganglia. Individuals with a PD diagnosis experience motor symptoms (e.g., tremors) and nonmotor symptoms (e.g., cognitive decline). Previous studies suggest that progression of cognitive dysfunction in other neurologic populations can be predicted by cumulative head injuries. The study examined the association between lifelong number of head injuries and nonmotor outcomes (cognitive complaints, depression, and quality of life).
Methods:
Participants consisted of 3,483 individuals with PD diagnoses who were enrolled in the Fox Insight study. Participants completed a self-report questionnaire to quantify the number of head injuries experienced throughout life. Participants also completed measures of nonmotor outcomes (cognitive complaints, depression, and quality of life) every 6 months over a 3-year period.
Results:
Cognitive complaints were more common among those experiencing more head injuries. Further, more severe depression and greater difficulties in quality of life were reported among individuals experiencing a greater number of head injuries. Additional analyses revealed the effect between cognitive complaints and number of head injuries was driven by individuals who experienced five or more head injuries in their lifetime.
Conclusions:
Among individuals with PD, a patient report of past head injuries may have prognostic implications for important nonmotor outcomes. Report of multiple head injuries may be particularly concerning.
Parkinson’s disease (PD) is a neurodegenerative movement disorder associated with reduced production of dopamine-producing cells and dysfunction of the basal ganglia. Approximately 1% of individuals over the age of 60 are affected by PD (1). Furthermore, PD is characterized by motor symptoms (e.g., tremors, bradykinesia) and nonmotor symptoms (e.g., mood symptoms, cognition).
Nonmotor symptoms are common in PD, such as constipation, hyposmia autonomic dysfunction, anxiety, depression, and cognitive complaints (2). Depression and cognitive impairments may be particularly burdensome in terms of decreased quality of life, caregiver burden, and the fact that these neuropsychiatric symptoms are commonly underrecognized and undertreated (2–5). Cognitive complaints include difficulties with organization and problem solving (executive dysfunction), attention, working memory, and visuospatial functioning (6). Approximately 25.6% of individuals newly diagnosed with PD report some degree of cognitive impairment (7). Degrees of cognitive impairment among individuals with PD range from mild (individuals remain functional in day-to-day life) to severely debilitating (termed Parkinson’s disease dementia).
Head injury refers to any injury to the head; traumatic brain injury (TBI) is a subtype of head injury characterized by changes in brain function or structural damage to the brain because of external force. TBI can lead to death, disability, and complications later in life (8). The association between repeated head injuries and a Parkinsonian or motor syndrome dates back to the early 1900s with references to “punch drunk syndrome” (9, 10). Early reports highlight the importance of damage to the substantia nigra, a region also shown to be degenerated in PD. Indeed, a recent meta-analysis found evidence that a history of TBI is associated with increased risk of PD (11).
TBIs also affect several cognitive domains: executive functioning, processing speed, attention, and long-term memory. Past research indicates that cognitive complaints frequently diminish within a couple of months following the head injury; however, chronic complications can arise (12–14). For instance, Broadway et al. (14) investigated delayed memory deficits in two populations who had experienced mild TBIs: those in the chronic phase (individuals reporting symptoms persisting for ≥3 months after TBI) and those in the subacute phase (injured ≤14 days from study entry, with or without symptoms). Broadway et al. (14) reported that individuals who had experienced chronic TBI recalled 15% fewer words than healthy controls, and individuals who had experienced subacute TBI recalled 18% fewer words than healthy controls. Additionally, individuals who had experienced a TBI performed worse on semantic clustering tasks and exhibited worse executive dysfunction than healthy controls. These findings suggest that cognitive symptoms such as word recall and executive functioning may persist for months after the incident.
TBIs have been shown to lead to more severe cognitive complaints among individuals with PD. Schiehser et al. (15) conducted a longitudinal study examining cognitive changes among individuals diagnosed with PD who had a history of TBI. Twenty-five PD patients who had experienced TBI were compared with 25 PD patients with no prior TBI history. Cognition, mood, and motor functioning were documented at baseline and at a 2-year follow-up. At baseline, there was no difference in cognitive ability between the two groups; however, at the 2-year follow-up, Schiehser et al. (15) found that individuals with PD and a history of TBI had significantly greater rates of cognitive impairment compared with PD patients with no history of TBI. To further complicate the situation, individuals with PD who had TBI significantly declined on objective cognitive tests over the 2-year period, whereas PD patients without TBI improved. Results from Schiehser et al. (15) indicate that a history of TBI may accelerate the degree of cognitive impairment in PD. Naturally, the consequences of PD vary from individual to individual; however, additional risk factors such as TBIs may escalate the severity of cognitive impairment.
Whereas TBIs are defined by changes in brain function or damage of the brain as a result of external force (8, 16), subconcussive injuries are head injuries sustained from blunt force without acute symptoms (17). Subconcussive head injuries are commonly experienced by players in sports such as soccer and American football (17). Recent studies have suggested that subconcussive injuries have long-lasting effects on cognition and brain structure. For instance, worsened performance in memory and attention have been observed among patients with subconcussive injuries (18). Furthermore, Koerte et al. (17) discovered cortical thinning among soccer players, which subsequently was associated with diminished cognitive performance compared to healthy controls. Along with evidence of cortical thinning among soccer players, risk of depression and impaired executive functioning was reported among American football players with a history of repetitive subconcussive injuries (19). Repetitive subconcussive injuries illustrate risks for future wellbeing and functioning (20). Specifically, subconcussive injuries have been associated with future risk of neurodegenerative disorders such as PD and Alzheimer’s disease (20). Animal studies have suggested that instances of head injury increase the levels of tau development (21) and influence the integrity of the blood-brain barrier (18). These findings suggest that head injuries may lead to an increased risk for developing neurologic compromise later in life.
Despite preliminary evidence that TBIs seemingly lead to more severe cognitive problems among individuals diagnosed with PD (15) limited research is available. Additionally, other important nonmotor outcomes, such as depression and quality of life (QoL), have not been investigated. To further elaborate on existing literature, we investigated the cumulative effects of lifelong head injuries in a large longitudinal cohort of individuals with PD. The first aim of this study was to examine whether the number of lifelong head injuries predicted cognitive complaints over 3 years. The second aim was to examine whether the number of lifelong head injuries predicted depression and QoL over 3 years. A third aim was to examine whether cognitive complaints could be predicted by threshold effects of the number of head injuries (i.e., how many head injuries are needed before differences in cognitive complaints are detected?) and severity of head injury as measured by duration of loss of consciousness (LOC).
Methods
Participants and Procedure
Data used in the preparation of this study were obtained on October 2, 2020, from the Fox Insight database, an online longitudinal cohort study investigating the progression of PD within a cohort of individuals. (For up-to-date information on the study, visit https://foxinsight-info.michaeljfox.org/insight/explore/insight.jsp.) Research was completed in accordance with the Helsinki Declaration. This study was approved by the Institutional Review Board at California State University San Bernardino.
The study consisted of a subset of 3,483 individuals with a diagnosis of PD who completed questionnaires about cognitive complaints and lifetime head injuries. The lifetime head injury questionnaire was completed at baseline only; all other questionnaires were completed every 6 months over the course of 3 years.
Measures
Lifetime head injuries.
The Head Injury or Concussion section of the Environmental Exposure Questionnaire is a self-reported measure retrospectively quantifying the number of head injuries experienced in a lifetime and the duration of LOC. Participants indicated the number of lifetime head injuries on a 6-point Likert-type scale ranging from 1, none; to 6, five or more. Duration of LOC was rated on a 4-point Likert-type scale (1, less than 5 minutes; 2, 5–59 minutes; 3, 1–24 hours; 4, longer than 1 day).
Cognitive complaints.
Participants completed the 15-item Penn Parkinson’s Daily Activities Questionnaire (22) to measure the extent to which they could complete cognitive tasks (e.g., reading, keeping track of time). Participants responded using a 5-point Likert-type scale with higher scores indicating greater ability to complete cognitive tasks (i.e., fewer complaints). This measure was completed every 6 months.
Depression.
Depressive symptoms were measured using the Geriatric Depression Scale—Short Form (23). Participants reported the presence or absence of symptoms for each item.
Quality of life.
The eight-item Daily Living Questionnaire (24) measured QoL. Participants used a 5-point Likert-type scale (0, never; 4, always or cannot do at all) to describe difficulty with various tasks (e.g., going out in public, getting dressed).
Motor symptoms.
Motor symptom severity was measured using part III (motor subscore) of a standard, well-validated measure, the Unified Parkinson’s Disease Rating Scale (25).
Statistical Analyses
Data were analyzed via multilevel modeling in SPSS version 25; standardized estimates are reported. The first aim investigated the influence of cumulative head injuries on cognitive complaints among individuals with PD. Cognitive complaint was entered as the outcome. Predictors included lifelong number of head injuries, gender, age, occasion (i.e., baseline, 6-month follow-up, … 36-month follow-up) and motor severity. The second aim examined whether number of head injuries predicted depression and QoL among individuals with PD. Two separate analyses were conducted: one with depression entered as the outcome, the other with QoL entered as the outcome. Predictors were similar to aim 1. The third aim examined whether cognitive complaints could be predicted by potential threshold effects of the number of head injuries and severity of head injuries as measured by duration of LOC. Cognitive complaints were entered as the dependent variable in both models. To examine threshold effects of the number of head injuries, the number of head injuries was treated as a categorical variable instead of as a continuous variable. Individuals reporting no head injuries were set as the reference group (e.g., no head injuries vs. one head injury, no head injuries vs. two head injuries, … no head injuries vs. five or more head injuries). In the second model, duration of LOC for the most severe head injury was entered as a predictor. Additional predictors included age, gender, duration, and motor severity.
Results
Demographic Characteristics
Participants consisted of 3,483 individuals newly diagnosed as having PD. Baseline sample characteristics are provided in Table 1. Among the participants reporting a head injury, the median duration between the head injury and date of enrollment in the study was 35.9 years (interquartile range=14.4–50.1 years).
| Variable | N | % | Range |
|---|---|---|---|
| Age (M±SD) | 64.9±8.7 | 28–91 | |
| Male | 50.9% | ||
| Cognitive complaints (M±SD) | 51.5±8.9 | 0–65 | |
| Depression (M±SD) | 3.7±3.5 | 0–15 | |
| Quality of life (M±SD) | 6.2±4.9 | 0–31 | |
| Motor severity (M±SD) | 24.3±7.6 | 1–63 | |
| Number of head injuries (M±SD) | 1.9±1.2 | 1–5 | |
| Individuals experiencing | |||
| 0 head injuries | 1,602 | 46 | |
| 1 head injury | 925 | 26.6 | |
| 2 head injuries | 539 | 15.5 | |
| 3 head injuries | 218 | 6.3 | |
| 4 head injuries | 84 | 2.4 | |
| 5 or more head injuries | 115 | 3.3 | |
| Individuals reporting LOCa | 668 | 19.2 |
TABLE 1. Baseline demographic and clinical information of 3,483 persons with new diagnosis of Parkinson’s disease
Aim 1: Cumulative head injuries as predictor of cognitive complaints.
Multilevel modeling was conducted to evaluate the impact of cumulative head injuries on cognitive complaints (Table 2). Analyses revealed that a greater number of lifetime head injuries significantly predicted greater cognitive complaints (Figure 1). More severe cognitive complaints were also associated with more severe motor symptoms, and cognitive complaints became more common as the study progressed.
| Parameter | B | SE | t | df | 95% CI | p |
|---|---|---|---|---|---|---|
| Number of head injuries | −0.04 | 0.02 | −2.30 | 2870 | −0.06, −0.01 | 0.021 |
| Motor symptom severity | −0.53 | 0.01 | −35.56 | 2896 | −0.56, −0.50 | <0.001 |
| Occasion | −0.08 | 0.01 | −17.54 | 2032 | −0.09, −0.07 | <0.001 |
| Age at baseline | −0.01 | 0.01 | −0.04 | 2890 | −0.03, 0.03 | 0.972 |
| Male gender | −0.02 | 0.03 | −0.59 | 2853 | −0.08, 0.04 | 0.552 |
TABLE 2. Predictors of cognitive complaints among persons with Parkinson’s diseasea
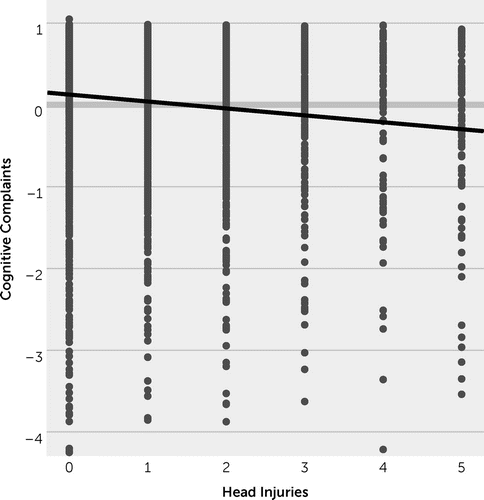
FIGURE 1. Association between cognitive complaints and number of head injuriesa
a Cognitive complaints depicted in a z-metric.
Aim 2: Cumulative head injuries as predictor of depression and QoL.
In regard to depression, more severe depressive symptoms were associated with a greater number of reported head injuries (Table 3; Figure S1 in the online supplement). More severe depression was also predicted by motor symptom severity and age at baseline.
| Parameter | B | SE | t | df | 95% CI | p |
|---|---|---|---|---|---|---|
| Number of head injuries | 0.04 | 0.02 | 2.71 | 2,873 | 0.01, 0.08 | 0.007 |
| Motor symptom severity | 0.43 | 0.02 | 26.33 | 2,802 | 0.40, 0.46 | <0.001 |
| Occasion | 0.04 | 0.02 | −1.67 | 2,032 | −0.01, 0.08 | 0.096 |
| Age at baseline | −0.16 | 0.02 | −9.56 | 2,805 | −0.19, −0.12 | <0.001 |
| Male gender | −0.02 | 0.03 | −0.48 | 2,793 | −0.08, 0.05 | 0.632 |
TABLE 3. Predictors of depressive symptoms among persons with Parkinson’s diseasea
In regard to QoL, a greater number of head injuries was associated with worse QoL (Table 4; Figure S2 in the online supplement). Worse QoL was also predicted by motor symptom severity, occasion, age at baseline, and male gender.
| Parameter | B | SE | t | df | 95% CI | p |
|---|---|---|---|---|---|---|
| Number of head injuries | 0.05 | 0.01 | 4.41 | 2,861 | 0.03, 0.07 | <0.001 |
| Motor symptom severity | 0.68 | 0.01 | 59.03 | 2,919 | 0.66, 0.70 | <0.001 |
| Occasion | 0.10 | 0.01 | 18.67 | 2,050 | 0.09, 0.11 | <0.001 |
| Age at baseline | −0.15 | 0.01 | −12.87 | 2,906 | −0.17, −0.13 | <0.001 |
| Male gender | −0.13 | 0.02 | −5.71 | 2,829 | −0.18, −0.08 | <0.001 |
TABLE 4. Predictors of quality of life among persons with Parkinson’s diseasea
Aim 3: Threshold effect and severity of head injuries.
To examine a threshold effect of the number of head injuries, we treated the number of head injuries variable as a categorical variable, with individuals reporting no head injuries set as the reference group (e.g., no head injuries vs. one head injury, no head injuries vs. two head injuries, … no head injuries vs. five or more head injuries). Analyses revealed that differences in cognitive complaints were only detected when individuals experienced five or more head injuries in their lifetime (standardized B=−0.20, p=0.018; Table S1 in the online supplement). There were no other significant group differences, suggesting that individuals who experienced fewer than five head injuries did not significantly differ in cognitive complaints from individuals without head injuries.
Additionally, we examined the association between cognitive complaints and the duration of LOC from the most severe head injury. The duration of LOC did not significantly predict cognitive complaints.
Discussion
This study demonstrated that cumulative head injuries predicted longitudinal cognitive complaints, depression, and QoL complaints among individuals with PD. Specifically, the association between cognitive complaints and head injuries was driven by individuals experiencing five or more head injuries in their lifetime. Overall, our findings supported our hypotheses that the cumulative number of lifetime head injuries has detrimental effects on nonmotor outcomes among individuals diagnosed with PD.
The findings support the limited past research investigating the effect of head injuries on PD symptomatology. In a small sample of individuals with PD (N=50), a history of mild to moderate TBI increased the risk of cognitive decline over a 2-year period (15). Specifically, declines were most prominent on tests of executive functioning, although there were trends for declines in memory. A cross-sectional study of 69 PD patients reported the number of TBIs reported by patients was correlated with performance on measures of executive function, memory, and language, as well as other important nonmotor outcomes including depression and QoL (26).
In contrast to the past literature that focused specifically on TBI among individuals with PD, the current study used the more inclusive category of head injuries. The fact that 54% of the sample reported a history of a head injury but less than 20% of the sample reported LOC raises the possibility that most head injuries reported were in the mild TBI or subconcussive range. Indeed, past studies using non-PD populations provide evidence that mild or subconcussive head injuries can have negative impacts on cognition and mood. Individuals who experienced mild closed head injuries are at an increased risk of major depression (27, 28) worsened QoL, and diminished cognition (18). Vasterling et al. (28) reported that veterans who endured head injuries exhibited greater depression symptoms than veterans who did not endure any head injuries. According to Maguen et al. (29), veterans who endured multiple head injuries were four times more likely to develop depression. Diminishment in memory, attention, and neuromotor abilities are associated with multiple subconcussive head injuries within sports populations (18). Among older adults, minor injuries are associated with cognitive impairment and difficulties with activities of daily living, particularly among frail older adults (30). Therefore, cumulative TBIs and head injuries may add to the worsened QoL, depression, and cognitive complaints experienced by individuals with PD.
There is debate over the neural mechanisms of cognitive impairment in PD and whether deficits are a frontal pattern of impairment (31, 32) or a posterior pattern of impairment (33). The traditional view suggests the neuropsychological profile in PD resides mainly within the frontostriatal circuitry, which profoundly affects cognition and mood, specifically poor performance in executive functioning tasks (e.g., planning, organization). More recently, there is appreciation for a posterior profile of cognitive impairment, which may include deficits in visuospatial functioning and predict PD dementia relatively early in the disease course (33). Ultimately, these views may not be mutually exclusive (34).
Regardless of the mechanisms underlying cognitive deficits in PD, TBIs have their own detrimental consequences to inflammation and white matter degeneration, which in turn affect cognition and increase risk for neurodegenerative disease (14, 20, 35). Johnson et al. (35) compared brain tissue of individuals who had survived between 10 hours and 27 years post-TBI with tissue of healthy age-matched controls. Overall, findings suggests that progressive neuroinflammatory processes can be triggered after a single moderate or severe TBI. Compared to healthy controls, brain tissues of individuals who had endured a single TBI were denser with microglia and phagocytic macrophages. The inflammation observed in the brain tissue of TBI individuals progressed for up to 18 years following the incident. The progression of inflammation in brain structures may explain cognitive deficits years after TBIs (14). Neural inflammation may also play a role in cognitive impairment in PD (36). Ultimately, future studies are needed to examine whether the association between head injuries and cognitive impairment in PD is mediated by neuroinflammatory markers. Additionally, a literature review by Graham and Sharp (37) discussed mechanisms that trigger neurodegeneration following TBIs. Graham and Sharp (37) described the influence of TBIs on abnormal tau, amyloid beta, and TDP-43, all of which are markers for Alzheimer’s disease. These proteins have been detected in individuals who had TBIs, and cognitive complications caused by the neurotoxicity of these proteins persisted for months and years after the injury. Moszczynski et al. (21) discovered similar results: abnormal tau was discovered within rats that experienced TBIs. Ultimately, TBI may trigger neurodegeneration due to its impact on brain anatomy.
This study was not without limitations. Cognition was assessed with a subjective measure, and future studies may benefit from objective neuropsychological tests. PD participants were asked to complete the questionnaire about cognitive complaints. It is possible that some participants may have had severe cognitive impairment and lack of awareness or anosognosia, which may limit the validity of their responses. Future studies may benefit from informant report in addition to self-report. As with most longitudinal studies of older adults, there is the possibility that additional neurologic compromise may be a confound. However, only 1.2% of the available sample reported experiencing a heart attack, stroke, or cranial or neurologic surgery, which may reduce concern about the potential for confounding neurologic conditions. Additionally, a differentiation between head injuries and TBI was not possible in the available data set.
Conclusions
Future studies may benefit from a more in-depth characterization of head injuries (e.g., alteration of consciousness, posttraumatic amnesia, acute neurologic change, penetrating vs. closed-head injury). Despite the study’s limitations, findings suggest that a patient report of past head injuries, particularly a report of multiple head injuries, may have prognostic implications for important nonmotor outcomes in PD.
1. : Epidemiology of Parkinson’s disease. J Neural Transm 2017; 124:901–905Crossref, Medline, Google Scholar
2. : Non-motor features of Parkinson disease. Nat Rev Neurosci 2017; 18:435–450Crossref, Medline, Google Scholar
3. : Anxiety and depression are better correlates of Parkinson’s disease quality of life than apathy. J Neuropsychiatry Clin Neurosci 2015; 27:213–218Link, Google Scholar
4. : Factors of importance to the caregiver burden experienced by family caregivers of Parkinson’s disease patients. Aging Clin Exp Res 2002; 14:371–377Crossref, Medline, Google Scholar
5. : Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2002; 8:193–197Crossref, Medline, Google Scholar
6. : Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012; 27:349–356Crossref, Medline, Google Scholar
7. : Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005; 65:1239–1245Crossref, Medline, Google Scholar
8. : Neuropsychology of traumatic brain injury: an expert overview. Rev Neurol 2017; 173:461–472Crossref, Medline, Google Scholar
9. : Punch drunk. JAMA 1928; 91:1103–1107Crossref, Google Scholar
10. : Boxing and the brain. BMJ 1989; 298:105–109Crossref, Medline, Google Scholar
11. : Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013; 28:1222–1229Crossref, Medline, Google Scholar
12. : The spectrum of mild traumatic brain injury: a review. Neurology 2017; 89:623–632Crossref, Medline, Google Scholar
13. : Predictors of post concussive symptoms 3 months after mild traumatic brain injury. Neuropsychology 2012; 26:304–313Crossref, Medline, Google Scholar
14. : Executive function predictors of delayed memory deficits after mild traumatic brain injury. Cortex 2019; 120:240–248Crossref, Medline, Google Scholar
15. : Cognitive functioning in individuals with Parkinson’s disease and traumatic brain injury: a longitudinal study. Parkinsonism Relat Disord 2016; 30:58–61Crossref, Medline, Google Scholar
16. : Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91:1637–1640Crossref, Medline, Google Scholar
17. : Cortical thinning in former professional soccer players. Brain Imaging Behav 2016; 10:792–798Crossref, Medline, Google Scholar
18. : Effects of sub-concussion on neuropsychological performance and its potential mechanisms: a narrative review. Brain Res Bull 2020; 165:56–62Crossref, Medline, Google Scholar
19. : Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 2017; 34:328–340Crossref, Medline, Google Scholar
20. : Imaging chronic traumatic brain injury as a risk factor for neurodegeneration. Alzheimers Dement 2014; 10:S188–S195Crossref, Medline, Google Scholar
21. : Pathologic Thr175 tau phosphorylation in CTE and CTE with ALS. Neurology 2018; 90:e380–e387Crossref, Medline, Google Scholar
22. : The Penn Parkinson’s Daily Activities Questionnaire-15: psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson’s disease. Parkinsonism Relat Disord 2016; 25:21–26Crossref, Medline, Google Scholar
23. : The geriatric depression scale (GDS). Best Practices in Nursing Care to Older Adults 2012; 4:1–2Google Scholar
24. : The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol Health 1997; 12:805–814Crossref, Google Scholar
25. : Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23:2129–2170Crossref, Medline, Google Scholar
26. : The impact of traumatic brain injury on cognitive and neuropsychiatric symptoms of Parkinson’s disease. Int Rev Psychiatry 2020; 32:46–60Crossref, Medline, Google Scholar
27. : Major depression in patients with closed head injuries. Neuropsychology 1987; 1:7–9Crossref, Google Scholar
28. : Head injury as a predictor of psychological outcome in combat veterans. J Trauma Stress 2000; 13:441–451Crossref, Medline, Google Scholar
29. : The impact of head injury mechanism on mental health symptoms in veterans: do number and type of exposure matter? J Traumatic Stress 2012; 25:3–9Crossref, Medline, Google Scholar
30. : Decline in activities of daily living after a visit to a Canadian emergency department for minor injuries in independent older adults: are frail older adults with cognitive impairment at greater risk? J Am Geriatr Soc 2015; 63:860–868Crossref, Medline, Google Scholar
31. : Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease; in Behavioral Neurology of Movement Disorders. Edited by Weiner WJ, Lang AE. New York, Raven Press, 1995, pp 85–95Google Scholar
32. : A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 2003; 16:193–210Crossref, Medline, Google Scholar
33. : The distinct cognitive syndromes of Parkinson’s disease: 5-year follow-up of the CamPaIGN cohort. Brain 2009; 132:2958–2969Crossref, Medline, Google Scholar
34. : Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis 2013; 11:79–92Crossref, Medline, Google Scholar
35. : Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013; 136:28–42Crossref, Medline, Google Scholar
36. : Senescence and inflammatory markers for predicting clinical progression in Parkinson’s disease: the ICICLE-PD study. J Parkinsons Dis 2020; 10:193–206Crossref, Medline, Google Scholar
37. : Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 2019; 90:1221–1233Crossref, Medline, Google Scholar



