Higher Health Service Costs Associated With Delayed Diagnosis of Functional Neurological Disorder
Abstract
Objective:
Functional neurological disorder (FND) is frequently encountered in clinical practice but commonly misdiagnosed, which might lead to higher direct costs for the health care system. The investigators analyzed the direct costs associated with the diagnosis of FND compared with costs associated with other neurological conditions and explored possible cost trends related to the clinical and demographic features of FND.
Methods:
Consecutive patients attending a general neurology clinic were recruited and underwent a structured assessment aimed to collect information pertaining to their demographic and clinical characteristics, as well as data regarding their prior diagnostic processes (e.g., the number of consulted specialists, number and type of investigations, emergency department visits, etc.). The costs were hence calculated and compared between the study groups.
Results:
A total of 155 consecutive patients were recruited; of these, 18.6% had FND, 55.84% had one or more other neurological disorder (OND), and 27.10% presented with comorbid FND and OND. The total prediagnostic costs (in euros [€]) were higher in the FND group compared with the OND group (median=€289, interquartile range [IQR] €385 vs. median=€98, IQR €216; Mann–Whitney U=879.5, p=0.04). There was a higher diagnostic delay in the FND group compared with the OND group (median=48 months, IQR 60 months vs. median=12 months, IQR 6 months; Mann–Whitney U=162.00, p<0.01). Diagnostic delay significantly correlated with the total costs in the entire study sample (Spearman’s ρ=0.25, p=0.003) but more strongly in the FND group (Spearman’s ρ=0.81, p<0.001). In the FND group, higher numbers of investigations and costs were associated with the presence of a physiological or psychological trigger and multiple symptoms.
Conclusions:
Delayed diagnosis of FND significantly affects health care system costs, and raising awareness about FND to improve the diagnostic process and outcomes is necessary.
Functional neurological disorder (FND) is characterized by symptoms that are internally inconsistent and incompatible with other neurological conditions (1). It has been estimated that about 15%–30% of patients who are admitted to neurology wards or seen in neurology clinics have FND (2–4). Despite its frequency, FND is often misdiagnosed (5), an issue that is further complicated by the fact that FND may frequently overlap with other neurological disorder (OND) (6).
There is limited information about health care use and costs among patients with FND. Preliminary evidence stemming from conditions that may be associated with FND, such as somatization disorder (7–9), has shown an estimated annual cost of up to £18 billion (approximately €21.5 billion); of which, inpatient hospital stays may account for £600 million (approximately €720 million) (8). Studies focusing on functional seizures have shown estimated annual costs to be between €8,000 and €21,000 per patient, including costs for diagnosis and treatment (10, 11). In addition, preliminary findings by Russell et al. (12) demonstrated that intensive short-term dynamic psychotherapy as a treatment for functional seizures could reduce health care costs (i.e., physician visits, physician costs, hospital admissions, and overall hospital costs) by more than 80%, emphasizing that correct diagnosis and proper management of this condition can lead to health care savings, along with improvements in emotional well-being. More recently, it was found that expenditures attributable to inpatient care for FND could be about $1.2 billion (approximately €1 billion) per year (13). Specifically, Stephen et al. (13) evaluated resource use and expenses for FND by assessing both emergency department evaluations and hospitalizations. The investigators calculated the annual costs of emergency department visits to be $163 million (approximately €143 million) for FND, a figure comparable to that for refractory epilepsy (13). These emergency department visits more frequently resulted in inpatient admissions and higher workup rates for FND than admissions for comparable neurological diagnoses (13).
However, in most of the aforementioned studies, the overall expenses of FND were calculated, including those associated with management of FND symptoms—which may be demanding and costly, especially when performed during inpatient stays—or emergency department visits were used as the only source of expenditure, dismissing alternative costs that FND patients may incur on an outpatient basis. In theory, one might expect that the costs associated with the prediagnostic workup may greatly affect the entire cost burden, given the high rate of misdiagnosis, with patients undergoing a number of unnecessary investigations and thus experiencing a significant diagnostic delay (5), which in turn has a detrimental effect on long-term outcomes (14). The costs associated with the diagnosis of FND are theoretically compressible, but there is only one recent study which focused on this aspect (15). It demonstrated high economic costs as a result of delayed diagnosis of FND patients recruited in a specialized clinic (15). However, this work focused on patients with a motor phenotype only and did not include a group of patients with OND as a comparator (15).
Here, we aimed to calculate the direct costs associated with the diagnosis of FND compared with that for OND to further confirm that these costs vary as a function of diagnostic delay. Additionally, we aimed to analyze possible cost trends associated with the clinical and demographic features of patients with FND to explore whether some clinical features/phenotypes (for example, the presence of multiple symptoms) could be associated with higher costs.
Methods
We conducted a prospective case-control study, recruiting consecutive patients who attended a general neurology outpatient clinic of one of the study authors (R. E.) during a 6-month period (January–June 2019). The study was approved by the local ethical committees, and all participants provided written informed consent to allow use of their anonymized data for research purposes. No exclusion criteria were applied.
All patients were evaluated by one author (R. E.) with significant expertise in FND who made the formal diagnosis of FND, OND, or comorbid FND and OND (CND). To collect demographic and clinical data, all patients underwent a customized, structured assessment (for further details, see the online supplement). The following clinical data were obtained: date of symptom onset (year), diagnosis date (year, if applicable), type of symptoms (motor, sensory, and cognitive, etc.), mode of presentation (acute/subacute vs. chronic), presence of a physiological or psychological trigger, and presence of additional symptoms (including fatigue, mood disturbances, sleep disorders, and neurovegetative symptoms). In our health system in Italy, nonemergent access to services is regulated according to the referral assigned by the general practitioner, and thus urgent visits have to take place within 72 hours from the time of the referral, whereas elective visits can take place beyond 72 hours from the time of the referral. We collected data regarding the type of referral (urgent vs. elective), as well as data related to any previous diagnostic process (the number of consulted specialists, including all medical specialists seen in the past for the same symptoms, the number and type of investigations, such as blood tests, imaging, and electrophysiological tests, emergency department access, and the number of prescriptions). This information was collected by directly asking patients about previous observations and diagnoses, as well as by reviewing all available medical records. The costs were then calculated by using official data from the Italian Ministry of Health for emergency department services and Campania Region (Tariff for Specialist Outpatient Services) for outpatient services (15). These costs are nearly equivalent to those assigned by the U.S. Centers of Medicare and Medicaid Services, with the only difference being that the Italian health program covers all Italian citizens, regardless of age, presence of disability, and income.
The Mann–Whitney U test was conducted for continuous variables because normality distribution was violated for many variables, whereas the chi-square test was used for categorical variables, with a p value <0.05 being deemed significant. These analyses were used to compare costs between FND and OND; to assess costs among subgroups of FND patients stratified by sex or specific clinical features (mode of presentation, presence of single vs. multiple symptoms, and presence of a physiological or psychological trigger); and to compare costs between OND and CND to explore the impact of comorbid FND. Finally, Spearman’s test was used to check for possible correlations between the diagnostic delay and the total costs incurred prior to a diagnosis. All analyses were performed with SPSS, version 20.0 (SPSS, Chicago).
Results
A total of 155 patients were recruited; of these, 28 patients (18.06%) had FND, none of whom reported having received a previous diagnosis of FND; 85 (54.84%) had OND; and 42 (27.10%) had CND. In the FND sample, 12 of 28 patients (42.85%) presented with a single symptom; specifically, four patients had functional seizures (33.34%), four had functional painful syndromes (33.34%), two had functional cognitive symptoms (16.66%), one had functional tremor (8.33%), and one had functional sensory symptoms (8.33%). Among the remaining 16 FND patients (57.15%), the following multiple symptoms were reported: pain, reported by seven patients (43.75%); sensory and cognitive symptoms, reported by five patients each (31.25%); weakness, reported by four patients (25.0%); paroxysmal disorder consistent with psychogenic nonepileptic seizures, reported by two patients (12.5%); gait/balance disorder and tremor plus speech (articulatory type) disorders, reported by two patients each (12.5%); and abnormal posturing, reported by one patient (6.25%). For further analyses, we grouped together motor symptoms (i.e., weakness, functional tremor, speech disorders, and dystonia). In the OND sample, 24 patients (28.2%) had neuromuscular disorders, 16 (18.8%) had movement disorders, 15 (17.6%) had vascular disorders, 14 (16.5%) had cognitive deficits, 10 (11.8%) had epileptic disorders, and six (7.1%) had demyelination disorders. Table 1 provides details of the gathered data in the three groups.
| Variable | FND (N=28) | OND (N=85)b | Comorbid FND and OND (N=42) | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Age (years) | 47* | 18 | 63 | 28 | 60 | 28 |
| Diagnostic delay (months) | 48* | 60 | 12 | 6 | 12 | 7.5 |
| Number of consulted specialists | 1* | 4 | 1 | 1 | 1 | 2 |
| Number of performed investigations | 2* | 4 | 1 | 2 | 1 | 1 |
| Number of emergency department visits | 1* | 4 | 1 | 1 | 0 | 1 |
| Number of prescriptions | 3* | 6 | 2 | 2 | 1 | 2 |
| Prediagnostic costs | ||||||
| Specialist visits (€) | 61.5* | 103.59 | 40.6 | 72.31 | 42.7 | 41.32 |
| Investigations (€) | 83.4* | 96.91 | 25.8 | 30.33 | 21 | 40.15 |
| Emergency department visits (€) | 108.4* | 197.29 | 29.09 | 151.01 | 32.3 | 144.6 |
| Prescriptions (€) | 40* | 80 | 0 | 40 | 0 | 40 |
| N | % | N | % | N | % | |
| Type of symptoms | ||||||
| Motor disorder (weakness, tremor, speech disorders, and dystonia) | 10 | 35.7 | 34 | 40.0 | 13 | 31.0 |
| Sensory disorder | 6 | 21.4 | 15 | 17.6 | 6 | 14.3 |
| Cognitive disorder | 7 | 25.0 | 17 | 20.0 | 8 | 19.0 |
| Gait and balance disorders | 2 | 7.1 | 9 | 10.6 | 4 | 9.5 |
| Epileptic disorder | 6 | 21.4* | 5 | 5.9 | 0 | 0.0 |
| Pain disorder | 11 | 39.3** | 18 | 21.2 | 22 | 52.4* |
| Additional symptoms | ||||||
| Fatigue | 3 | 10.7** | 2 | 2.4 | 2 | 4.8 |
| Mood disturbances | 4 | 14.3* | 2 | 2.4 | 0 | 0.0 |
| Sleep disorders | 1 | 3.6 | 2 | 2.4 | 4 | 9.5 |
| Neurovegetative symptoms | 3 | 10.7 | 5 | 5.9 | 2 | 4.8 |
| Single/multiple symptoms | ||||||
| Single | 12 | 42.9* | 61 | 71.8 | 28 | 66.7 |
| Multiple | 16 | 57.1* | 24 | 28.2 | 14 | 33.3 |
| Trigger | ||||||
| Presence of a trigger | 6 | 21.4** | 7 | 8.2 | 3 | 7.1 |
| Physical | 3 | 10.7 | 6 | 7.1 | 4 | 9.5 |
| Psychological | 3 | 10.7* | 1 | 1.1 | 1 | 2.4 |
| Mode of presentation | ||||||
| Acute/subacute | 13 | 46.4 | 34 | 40.0 | 17 | 40.5 |
| Chronic | 15 | 53.6 | 51 | 60.0 | 25 | 59.5 |
| Referral type | ||||||
| Urgent | 11 | 39.3 | 44 | 51.8 | 18 | 42.9 |
| Elective | 17 | 60.9 | 41 | 48.2 | 24 | 57.1 |
TABLE 1. Demographic and clinical characteristics and prediagnostic costs between patients with functional neurological disorder (FND), other neurological disorder (OND), and comorbid FND and ONDa
There was a significant difference between the FND and OND groups with regard to age (FND: median age=47 years, interquartile range [IQR]=18; OND: median age=63 years, IQR=28; Mann–Whitney U=670.5, p=0.001; Table 1). Patients with FND had a higher proportion of epileptic disorders compared with patients in the OND group (21.4% vs. 5.9%, respectively; χ2=5.79, p=0.02; Table 1). Mood symptoms (14.3% vs. 2.4%; χ2=5.97, p=0.02; Table 1) and multiple symptoms (57.0% vs. 28.0%; χ2=7.70, p=0.006; Table 1) were significantly more common in the FND group compared with the OND group, respectively. In addition, 21.4% of patients with FND reported a physiological or psychological trigger compared with 8.2% of patients with OND (p=0.06). Among patients with FND, 10.7% reported a physiological trigger (e.g., surgery, injury, infectious disease), and 10.7% reported a psychological trigger (e.g., grief, job loss). Presence of a psychological trigger was significantly higher in the FND group compared with the OND group (10.7% vs. 1.1%, respectively; χ2=4.73, p=0.04; Table 1).
There was a significant association between mode of presentation and type of referral in the OND group (69.7% of patients with an acute/subacute presentation received referrals for urgent visits, and 61.2% with chronic disorders received referrals for elective visits; χ2=7.55, p=0.006). However, this association was not significant in the FND group (χ2=1.68, p=0.19), with 54.4% of FND patients with an acute/subacute presentation receiving referrals for elective visits.
The diagnostic delay could be reliably collected for 15/28 (53.6%) of FND patients. There was a significantly higher diagnostic delay in the FND group compared with the OND group (median=48 months [IQR=60] vs. 12 months [IQR=6], respectively; Mann–Whitney U=162.00, p<0.001). Compared with the OND group, patients with FND had a higher number of specialist visits (Mann–Whitney U=875.5, p=0.04; Table 1), investigations (Mann–Whitney U=817.5, p=0.02; Table 1), emergency department visits (Mann–Whitney U=867.5, p=0.046; Table 1), and prescriptions (Mann–Whitney U=892.5, p=0.04; Table 1), which resulted in total higher costs prior to receiving their diagnosis (FND vs. OND: median=€289, IQR €385 vs. median=€98, IQR €216, respectively; Mann–Whitney U=879.5, p=0.04; Figure 1). Patients’ health system costs are summarized in Table 1.
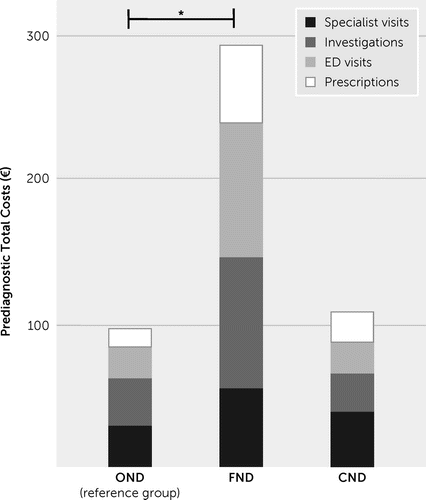
FIGURE 1. Costs incurred prior to the diagnosis of functional neurological disorder (FND) among 155 consecutive patients attending a general neurology clinica
aFor graphical purposes, mean values are presented with the statistical significance referring to nonparametric tests (for further details, see the article text). CND=comorbid FND and other neurological disorder (OND); ED=emergency department. *p<0.05.
FND patients with the presence of a physiological or psychological trigger had higher specialist consultation costs compared with those without a trigger (median: €104 [IQR: €103] vs. €21 [IQR: €103]; Mann–Whitney U=29.50, p=0.04). FND patients with multiple symptoms underwent a higher number of investigations compared with those with a single symptom (median: 3 [IQR: 3] vs. 1 [IQR: 2]; Mann–Whitney U=52.50, p=0.04). There were no significant differences in economic outcomes for FND with regard to sex, referral type (i.e., urgent vs. elective), or mode of symptom presentation (p>0.05).
With the exception of pain disorder, which was higher in the comorbid group compared with the OND group (Table 1), there were no differences between these groups with regard to the collected clinical variables or costs (Table 1, Figure 1).
In the entire study sample, there was a significant correlation between diagnostic delay and higher total costs incurred prior to receiving a diagnosis (Spearman’s ρ=0.25, p=0.003), but this correlation was stronger in the FND group (Spearman’s ρ=0.81, p<0.001) and not significant in either the OND (Spearman’s ρ=0.16, p=0.14) or comorbid (Spearman’s ρ=−0.09, p=0.54) group.
Discussion
In this study, 18% of consecutive patients referred to our general neurology outpatient clinic were diagnosed with FND, which is in line with previous observations (2, 4). It is noteworthy that none of our patients received a previous FND diagnosis elsewhere, which may have been a result of the relatively small sample size, but this more strongly points toward failure to recognize FND. Previous studies have in fact demonstrated that up to 73.0% of patients with FND receive one or more misdiagnoses of OND, with a mean diagnostic delay of about 6 years (5). This is corroborated by the present results showing a significantly higher diagnostic delay (of about 4 years) in our FND sample. Importantly, we also found that functional symptoms were present in about 33.0% (42/127) of patients with OND, which includes those with OND comorbid with FND, and this reinforces the evidence that FND and OND can frequently overlap (6).
Patients with FND more frequently exhibited multiple symptoms and mood disorders, which is in line with previous studies (16–18). We acknowledge that the presence of mood disorders was recorded on the basis of clinical interview only and not by means of validated instruments, which may have led to an underestimation of their prevalence. However, mood disorders may have also been underestimated in the OND and comorbid groups, given evidence that depression and subthreshold depressive symptoms are highly prevalent in ONDs such as Parkinson’s disease and multiple sclerosis. There was a tendency for fatigue and pain symptoms to be present. These symptoms are frequently found in FND (16, 19, 20) and have been deemed to negatively influence long-term prognosis (21). Again, this may have been due to the relatively small sample size.
Interestingly, we found a significant association between the mode of presentation and the type of referral made by general practitioners, with about 55.0% of FND patients being referred for elective visits. This may have been a result of the erroneous perception of FND as a condition that is not urgent to treat. A recent survey demonstrated that one of the most common approaches among general practitioners in managing FND is to “wait to see how symptoms develop” (22). This contrasts with evidence that delayed diagnosis is a significant risk factor for poor outcome at follow-up evaluations (14).
Regarding the main aim of the present study, we found that the direct costs incurred prior to the diagnosis of FND was threefold greater than that for OND. This is in line with a body of work showing higher costs associated with FND (11, 13), as well as with somatization disorder (7). The main difference between these prior findings and our data is that we strictly focused on the costs incurred prior to a diagnosis, and therefore we did not include management costs. This difference is important because the costs we calculated could be significantly reduced. The optimization of the diagnostic process in FND may therefore result in a reduction of the economic burden for the health care system, as well as in the reduction of time needed for a diagnosis, with important prognostic implications. Our results confirm and expand on previous observations of patients with FND with a motor phenotype (15) and call for educational programs targeting primarily general practitioners, as well as other medical specialists who may encounter these patients, in order to increase knowledge about this condition. In fact, more detailed cost analysis in our FND group showed a significant correlation with diagnostic delay, as well as that patients with trigger events had more specialist consultations, whereas those with multiple symptoms underwent more investigations, which may suggest lack of recognition of these features as part of the clinical spectrum. As recently suggested by Strom (23), it seems necessary to change the approach to the diagnosis of FND, which would involve avoiding unnecessary tests, therefore reducing the possibility of retraumatizing the patient and reducing the time required to correct misdiagnosis, enhancing the acceptability of such an approach. This concept was also emphasized by Perez et al. (24), who pointed toward the need for specific training to provide clinicians with the fundamental tools to recognize and manage FND symptoms (23, 25). We advocate for this training to begin when medical students are enrolled in their medical school programs. This call for early training should not only target neurologists and psychiatrists but clinicians in other medical specialties, because patients with FND have a great variety of symptoms and are often first referred to specialists in other medical disciplines (5).
Interestingly, the presence of FND among patients with OND did not affect health care system costs. This may have occurred because the development of functional symptoms may have attributed to the “main” neurological diagnosis, avoiding the need for other specialist consultations or investigations. Nonetheless, the high rate of comorbidity between FND and OND highlights the diagnostic challenge in distinguishing one from the other (5, 6), a distinction that is crucial for obvious therapeutic implications (26, 27).
Conclusions
In summary, our data show that the cost burden prior to receiving a diagnosis of FND is three times higher than that for OND due to significant diagnostic delays. A collaborative effort should be pursued in order to optimize the diagnostic process undergone by patients, not only to reduce costs but also time to reach a correct diagnosis, which would arguably improve outcome. This could be achieved by enhancing education and awareness about FND and OND among general practitioners and other medical specialists.
There are several limitations to this study. First, there was a relatively small sample size, which likely explains our statistical findings with regard to sex, fatigue, and pain. This also justified the grouping of patients in macro-categories (motor, sensory, and cognitive, etc.). Although this approach may be disputable, there is increasing evidence of a largely shared psychopathology between different FND phenotypes (1, 28), and we did not find differences among FND patients when stratified by phenomenology (data not shown). Second, recruitment was performed in a tertiary center, which may have created a selection bias toward patients with more difficult-to-treat symptoms (i.e., multiple symptoms) or a higher rate of CND, compared with previous studies (6). Nevertheless, a recruitment bias should also be true for patients with OND, thus minimizing the risk of inflated cost calculation. Third, for some analyses related to diagnostic delay, the data could be reliably collected only for about half of the patients in the FND group. We cautiously believe that this may be attributable to a type of recall bias, which is inherent to any self-report questionnaire or assessment tool and may be particularly problematic if the disorder of interest is hypothesized to involve underreporting through a pathological process, such as repression or dissociation (17, 29), and also when patients are not aware that the involvement of different systems is part of the same spectrum (that is, patients may report the onset of the newly developed symptom not acknowledging that their former symptoms are part and parcel of the same disorder).
Our data suggest that failure to promptly recognize FND and consequent diagnostic delay are crucial factors causing unnecessary specialist consultations, investigations, emergency department visits, and prescriptions, contributing to higher costs for the health care system.
1. : Psychogenic nonepileptic seizures and movement disorders: a comparative review. Neurol Clin Pract 2016; 6:138–149Crossref, Medline, Google Scholar
2. : Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol 2012; 259:33–38Crossref, Medline, Google Scholar
3. : Introductory textbook of psychiatry; in The American Psychiatric Publishing Textbook of Psychiatry. Washington, DC, American Psychiatric Association, 2014, pp 271–275Google Scholar
4. : Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112:747–751Crossref, Medline, Google Scholar
5. : Clinical correlates of functional motor disorders: an Italian multicenter study. Mov Disord Clin Pract 2020; 7:920–929Crossref, Medline, Google Scholar
6. : Functional motor disorders associated with other neurological diseases: beyond the boundaries of “organic” neurology. Eur J Neurol 2021; 28:1752–1758Crossref, Medline, Google Scholar
7. : Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005; 62:903–910Crossref, Medline, Google Scholar
8. : The cost of somatisation among the working-age population in England for the year 2008–2009. Ment Health Fam Med 2010; 7:71–84Medline, Google Scholar
9. : Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom 2012; 81:265–275Crossref, Medline, Google Scholar
10. : Improved health care resource utilization following video-EEG confirmed diagnosis of non-epileptic psychogenic seizures. Seizure 1998; 7:385–390Crossref, Medline, Google Scholar
11. : The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav 2014; 33:45–48Crossref, Medline, Google Scholar
12. : A pilot study of reduction in healthcare costs following the application of intensive short-term dynamic psychotherapy for psychogenic nonepileptic seizures. Epilepsy Behav 2016; 63:17–19Crossref, Medline, Google Scholar
13. : Assessment of emergency department and inpatient use and costs in adult and pediatric functional neurological disorders. JAMA Neurol 2021; 78:88–101Crossref, Medline, Google Scholar
14. : Psychogenic axial myoclonus: clinical features and long-term outcome. Parkinsonism Relat Disord 2014; 20:596–599Crossref, Medline, Google Scholar
15. : Economic costs of delayed diagnosis of functional motor disorders: preliminary results from a cohort of patients of a specialized clinic. Front Neurol 2021; 12:786126Crossref, Medline, Google Scholar
16. : Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018; 75:1132–1141Crossref, Medline, Google Scholar
17. : Attachment styles, identification of feelings and psychiatric symptoms in functional neurological disorders. J Psychosom Res 2021; 147:110539Crossref, Medline, Google Scholar
18. : Neuropsychiatric associations with gender, illness duration, work disability, and motor subtype in a US functional neurological disorders clinic population. J Neuropsychiatry Clin Neurosci 2017; 29:375–382Link, Google Scholar
19. : A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav 2005; 6:264–265Crossref, Medline, Google Scholar
20. : Psychogenic nonepileptic seizures and chronic pain: a retrospective case-controlled study. Epilepsy Behav 2012; 25:662–665Crossref, Medline, Google Scholar
21. : Neuropsychiatric factors linked to adherence and short-term outcome in a US functional neurological disorders clinic: a retrospective cohort study. J Neuropsychiatry Clin Neurosci 2018; 30:152–159Link, Google Scholar
22. : Functional neurological disorders as seen by a cohort of general practitioners in Northern Italy: evidence from an online survey. Front Neurol 2021; 12:583672Crossref, Medline, Google Scholar
23. : Functional neurologic disorders: bringing the informal and hidden curriculum to light. Neurol Clin Pract 2020; 10:471–472Crossref, Medline, Google Scholar
24. : Cautionary notes on diagnosing functional neurologic disorder as a neurologist-in-training. Neurol Clin Pract 2020; 10:484–487Crossref, Medline, Google Scholar
25. : The confluence of quality of care, cost-effectiveness, pragmatism, and medical ethics in the diagnosis of nonepileptic seizures: a provocative situation for neurology. Arch Neurol 2001; 58:2066–2067Crossref, Medline, Google Scholar
26. : Variables associated with co-existing epileptic and psychogenic nonepileptic seizures: a systematic review. Seizure 2016; 37:35–40Crossref, Medline, Google Scholar
27. : L-DOPA-induced off: functional overlay in Parkinson disease. J Neurol Sci 2016; 365:1–2Crossref, Medline, Google Scholar
28. : Functional motor phenotypes: to lump or to split? J Neurol 2021; 268:4737–4743Crossref, Medline, Google Scholar
29. : The relationship between types of life events and the onset of functional neurological (conversion) disorder in adults: a systematic review and meta-analysis. Psychol Med 2022; 52:401–418Crossref, Medline, Google Scholar



