Cranial Nerve Zero (CN 0): Multiple Names and Often Discounted yet Clinically Significant
Anatomical descriptions of the cranial nerves (CNs) in humans have been historically documented in numerous scientific reports, starting from the classical Greek manuscripts to today’s contemporary medicine (15). Our understanding of the anatomy of human CNs, including their functional components, progressed from 1500 to 1900 C.E. through cadaveric dissections. These dissections were followed by detailed anatomical and scientific reports conducted by anatomists and surgeons of that era (16). The historical records emerging from well-known anatomists, from Galen to von Sömmerring, led to the canonical classification of the 12 pairs of CNs acknowledged in most medical textbooks and the anatomical literature (15–17). The order and the anatomical descriptions assigned to each CN were inspired by each CN’s anatomical positioning (i.e., the relationship to other structures), functional relevance, and, in rare cases, appearance (15, 16, 18). Anatomically, CNs are peripheral nerves that exit the cranium or enter the cranium. They are designated by a Roman numeral (i.e., I–XII), as well as a functional, locative, or other nonnumeric name (e.g., olfactory: CN I; optic: CN II; oculomotor: CN III; trochlear: CN IV; trigeminal: CN V; abducens: VI; facial: CN VII; vestibulocochlear: VIII; glossopharyngeal: IX; vagus: X; accessory: XI; and hypoglossal: XII).
Interestingly, very little is known regarding cranial nerve zero (CN 0). CN 0 was initially known by other names, such as nervus terminalis, nerve of Pinkus, tractus olfacto-commissuralis, new nerve, terminal nerve, nerve nulla (i.e., nothing, zero), and cranial nerve 13 (1, 4, 9, 19). This enigmatic nerve was initially discovered in elasmobranchs (a subclass of Chondrichthyes, or cartilaginous fish) in 1878 by Fritsch, who described it using the term “supernumerary nerve.” At the time, the classification of CNs was already established by using Roman numeral symbols as noted above (1). This nerve has been described in a variety of species, including humans in 1905, and it is now usually referred to as CN 0 (1). This name is consistent with the nerve’s rostral display relative to the other CNs and thereby conforms to the preestablished numeric order of human CNs (19). Furthermore, CN 0 was the name officially designated in the Terminología Anatómica (issued in 1998 by the Federative Committee on Anatomical Terminology of the International Federation of Associations of Anatomists) (20, 21). CN 0 has been distinctly described in human brain of fetuses, infants, and adults (19). Subsequent studies have provided insights into its embryology, histological structure, neurophysiology, and clinical significance (9).
Embryology
CN 0 is one of the most enigmatic nervous system structures among vertebrates. Similar to other CNs, its embryological origins rest in synergistic developmental interactions between the neural crest and sensory placodes (22, 23). The neural crest is an important embryological structure comprising multipotent cells that differentiate into several cell types and contribute to many nervous system components (e.g., CNs, peripheral nerves, ganglia) and craniofacial tissues (e.g., cartilage and bones). Placodes are nervous tissue precursors that potentiate the development of sensory structures, such as the neurons of the nose that form the olfactory epithelium, the inner hair cells, and related tissues (24). The formation of CN 0 occurs at the frontmost boundary of the migrating neural crest cells, the termination of the neural tube, and at the adenohypophyseal and olfactory placodes (22). The embryological origin of CN 0 is similar to that of the rest of the human olfactory nerve structures, such as the olfactory nerves (CN I), the olfactory bulbs, and the vomeronasal organ (VNO) (1, 7, 25). It comprises one to two nerve bundles passing through the anterior end of the cribriform plate (7). Evidence suggests that these nerve fibers enter the brain along with the olfactory nerves and those of the vomeronasal processes at embryological stages 17 and 18 (i.e., Carnegie stages 17 and 18 of human embryonic development) (7, 26, 27). A recent embryological study employing classical histological techniques reported the identification of the VNO and CN 0 fibers in parasagittal sections of 7-week-old (19-mm) human embryos (1).
A unique and interesting feature of this nerve is its structural organization. In most species (including humans), the nerve is composed of axons expressing immunoreactivity to the decapeptide gonadotropin-releasing hormone (GnRH) (1, 4, 22). The GnRH neuroendocrine cells and the nerve fibers of CN 0 mainly arise from the olfactory placode with neural crest contributions (22, 28). However, the literature suggests that GnRH neurons may also arise from other embryological origins (23, 29–31). GnRH neurons of the hypothalamus differentiate and develop from outside the diencephalon into the forebrain, following a migratory route out of the placodal epithelium that carries the central fibers of CN 0, combined with CN I and the VNO (32). Faulty embryological processes (i.e., inadequate migration and genetic mutations) may result in reproductive issues and other physiological disturbances, including anosmia in some cases. Currently, some of the factors modulating GnRH neuronal migration are known, but the exact mechanisms regulating the migratory processes remain inconclusive (33).
Structure and Function
CN 0 is identifiable in human brain across neurodevelopmental stages, including in embryos, fetuses, children, and adults (2, 34–37). A recent study of human fetuses identified ganglion cell bodies along the crista galli of the ethmoid bone at 10 weeks (crown-rump length [CRL]: 76 mm) (1). Another study (30 fetuses at 7–18 weeks; CRL: 25–160 mm) reported its location at the anterior edge of the perpendicular lamina of the ethmoid bone. The bundles of fibers were observed running along and crossing posterior to the nasal branch of the anterior ethmoidal nerve (a branch of the ophthalmic division of CN V). Groups of small ganglionic cells by the crista galli, whose axons constitute most of CN 0, were also observed (7). In adults, the nerve keeps its plexiform structure and has ganglionic cells appearing in the ethmoid bone within the region of the cribriform plate. The nerve fibers appear to travel by the olfactory trigone close to the medial olfactory gyrus and lamina terminalis of the midbrain (1, 2) (Figure 1).
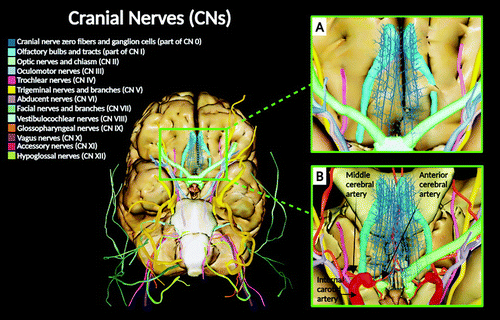
FIGURE 1. Cranial nerves. Panel A shows a digital depiction of a three-dimensional virtual dissection (basal view) of the CNs of the human brain, including the CN 0. Panel B shows a digital depiction emphasizing the CN 0 along with selected arteries of the Circle of Willis. The frontal lobes of the brain are dissected, as well as part of the optic nerve system (optic chiasm and left optic nerve) to illustrate the location of the CN 0 fibers traveling in the region where the anterior cerebral and middle cerebral arteries bifurcate from the internal carotid. In adults, CN 0 can be distinguished on the ventral surface of the brain (gyri recti area) by the frontal lobe, where the nerve fibers are traveling by the olfactory trigone close to the medial olfactory gyrus, along with the olfactory tracts and bulbs (1–6).
All images created under the terms of the Creative Commons Attribution License. Created with VH Dissector and BioRender.com.
The location and distribution of CN 0 within the nasal cavity and the cranium are distinct. In the nasal cavity, its fibers intermingle with those of the vomeronasal and olfactory nerves, respectively. However, CN 0 fibers travel medially compared with CN I fibers (1). CN 0 fibers can be distinguished from those of CN I and vomeronasal nerves on the basis of their anatomic characteristics. Whereas fibers of the CN 0 travel in the superior and anterior portions of the nasal cavity branching from its ganglionic cells, CN I fibers travel toward the olfactory bulb. In addition, the vomeronasal nerve fibers arise from the VNO (1, 7, 35, 36).
Intracranially, CN 0 fibers appear by the olfactory bulbs (over the dura mater) or embedded within the dura mater of the brain (37). CN 0 and the vomeronasal nerves penetrate and travel through the cribriform plate, where the plexiform fibers of CN 0 can be discerned along its ganglionic cell bodies over the cribriform plate surface (2, 6) (Figures 2, 3). Evidence suggests that these ganglionic cells appear as early as 6 weeks in human development (26). Ventrally on the brain surface and lateral to the olfactory bulbs, CN 0 and the vomeronasal nerves rejoin course along the CN I fibers. The vomeronasal and CN I fibers travel to the olfactory bulbs, where second-order neurons synapse (at the glomeruli), projecting to the vomeronasal and olfactory brain cortices, respectively (3). The CN 0 plexus travels parallel to the olfactory tracts in proximity to the septal region, close to the bifurcation of the anterior and middle cerebral arteries (Figure 1B). Its fibers are unmyelinated and travel next to the dura mater, passing into the subarachnoid space and finally connecting to the pia mater at the gyri recti of the frontal lobes (2, 37, 38). Some investigators contend that the precise anatomical location where CN 0 enters the brain is ambiguous (1). However, one of its initial monikers is the terminal nerve or nervus terminalis, implying that it enters the forebrain via the lamina terminalis (19).
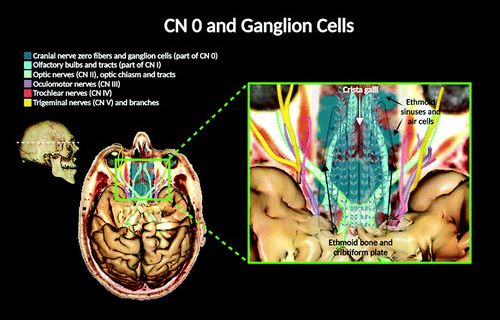
FIGURE 2. A digital depiction of a three-dimensional virtual dissection of the CNs (CNs 0–V). Superior view of the brain (frontal lobes removed) is illustrated. The CN 0 has many fibers exhibiting ganglionic cell bodies appearing over the ethmoid bone and within the region of the cribriform plate (1, 2, 6).
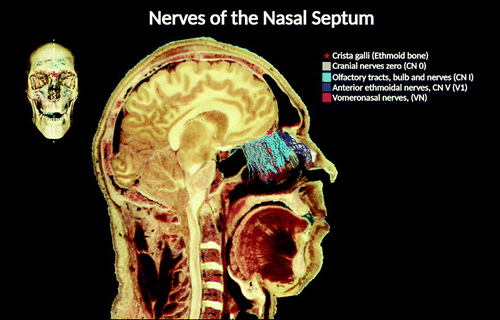
FIGURE 3. A midsagittal view of the head and neck, displaying the brain and the nerves of the nasal septum. The location and distribution of the CN 0 within the nasal cavity and the cranium are different. In the nasal cavity, CN 0 travels in proximity to the cribriform plate of the ethmoid bone, where its fibers cross rostrally from the brain into the olfactory epithelia (1, 4). The CN 0 plexus travels parallel to the olfactory tracts and bulbs in proximity to the septal region. The fibers from the vomeronasal nerves and CN 0 are intermingled, located medially to the olfactory nerves (1). The bundles of fibers run along and cross posterior to the nasal branch of the anterior ethmoidal nerve (a branch of the ophthalmic division of CN V) (7).
Despite the anatomical relationship of CN 0 to the vomeronasal nerve and CN I fibers, its function is distinct from that of both of these other structures. Given its physiological correlation with GnRH expression, CN 0 is functionally associated with the neuroendocrine regulation of reproductive behavior, among other functions (e.g., olfaction, autonomic and vasomotor regulation, paracrine secretion of nitric oxide, and immunologic defense mechanisms) (4, 25, 39). The axons of CN 0 support the migration of GnRH neurons to the hypothalamus, thereby potentiating the development of the hypothalamic-pituitary-gonadal axis in varied species, including humans (8, 23, 40, 41). In addition, the GnRH component of CN 0 has been suggested to be neuromodulatory, exerting a level of neurophysiological regulation over the olfactory epithelium, thus making pheromones more readily detectable (8).
The neuromodulatory role of CN 0 in reproductive behavior via GnRH poses a thought-provoking linkage with the hypothalamic “kisspeptin neuronal network” (KP) (9). Kisspeptin is a neuropeptide, encoded by KISS1, originally identified as a metastasis suppressor gene (42, 43). It binds to the kisspeptin receptor (KissR), which was first recognized as an orphan G-protein-coupled receptor-54 (44). The KP neural circuit is involved in the central endocrinological regulation of sexual development and human reproductive functions, essentially by inducing GnRH secretion from the hypothalamus. Physiologically, this network regulates the response of the gonadotropins, follicle-stimulating hormone, and luteinizing hormone, inducing the synthesis and release of the gonadal steroid hormones testosterone and estradiol. These hormones are essential for the physiology of reproductive behavior (12–14). Researchers hypothesize that CN 0 may trigger hormonal responses independently or together with other neurophysiological pathways of the brain, such as the KP neural circuit (1, 9) (Figure 4).
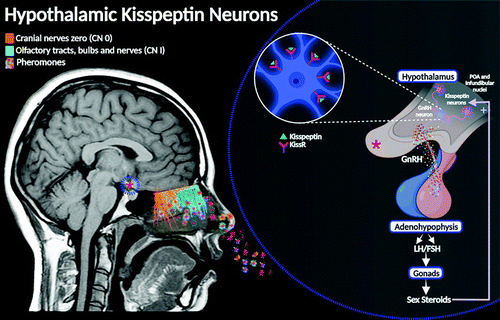
FIGURE 4. Hypothalamic kisspeptin neurons are shown. The gonadotropin-releasing hormone (GnRH) component of CN 0 has been suggested to be neuromodulatory, exerting a level of neurophysiological regulation over the olfactory epithelium, thus making pheromones more readily detectable (8). This neuromodulatory role poses a thought-provoking linkage with the hypothalamic “kisspeptin neuronal network” (KP) (9). In humans, KP-synthesizing neurons have been localized in two major hypothalamic sites: the preoptic (POA) and the infundibular areas (analogous to arcuate nucleus) (10, 11). The KP neural circuit is involved in the central endocrinological regulation of sexual development and human reproductive functions, essentially by inducing GnRH secretion from the hypothalamus. Consequentially, this causes a regulatory response over the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH), thereby inducing the synthesis and release of the gonadal steroid hormones, testosterone, and estradiol (12–14).
COVER. Digital depiction of a three-dimensional virtual dissection (basal view) of the cranial nerves (CNs) of the human brain, emphasizing cranial nerve zero (CN 0).
Clinical Implications
The normal development of the GnRH neural circuit is imperative for functional reproduction in vertebrates. In humans, any disturbances in the development of the nasal placode and/or migration of GnRH neurocircuitry may result in various forms of hypogonadism, including Kallmann syndrome. Kallmann syndrome is a rare genetic condition characterized by hypogonadotropic hypogonadism, typically causing lack of sexual development and anosmia (45, 46). Clinical studies have revealed that loss-of-function mutations in the kisspeptin 1 gene (KISS1) result in hypogonadotropic hypogonadism or precocious puberty (based on the type of mutation), indicating that kisspeptin plays an essential role in normal gonadotropin-releasing hormone physiology and in puberty (42, 47, 48).
In the central nervous system (CNS), the KISS1 gene is primarily expressed in the hypothalamus, functioning as an essential gatekeeper of the GnRH reproductive circuit, allowing the integration of central and peripheral inputs (42, 49). However, the KISS1 gene is expressed in other locations within the CNS; therefore, kisspeptin signaling is not restricted to the hypothalamic region (50). In humans, KISS1 has been identified in the amygdala, caudate, cingulate, globus pallidus, hippocampus, medial and superior frontal gyri, nucleus accumbens, parahippocampal gyrus, substantia nigra, putamen, and thalamus (50–53). In addition, the kisspeptin system participates in several circuits within the limbic system that mediate anxiety, fear, other negative emotions, and olfaction (52–55).
The role of kisspeptin in fear has not been explored in many species. However, some studies of zebrafish have presented preliminary evidence supporting the notion that kisspeptin can decrease fear responses possibly via the serotonergic circuit (56–58). The serotonergic system has also been investigated in order to examine kisspeptin’s modulatory functions in emotion. In rodents, intraventricular administration of kisspeptin (dose dependent) overturned immobility, climbing, and swimming frequency during an experimental swimming test, confirming its potential neuromodulatory actions (59).
Kisspeptin has also been implicated in anxiety and the reward pathways. Anxiety triggers stress hormones as regulated by the hypothalamic-pituitary-adrenal axis. However, this response is not affected by peripheral kisspeptin administration in animals or humans (55, 60). Interestingly, kisspeptin signaling in the hypothalamus is reduced by stress-induced plasma corticosterone in rodents, indicating a possible connection between the endocrinological aspects of anxiety and stress responses (53, 61). Additionally, kisspeptin appears to diminish the physiological effects of morphine, suggesting its possible involvement in the regulation of mesocorticolimbic dopaminergic activity (62, 63). The centers for reward and addiction are functionally related to kisspeptin expression (64). The dopaminergic projections connecting the ventral tegmental area and the nucleus accumbens have long been linked to the regulatory mechanisms of locomotion in rodents (65, 66). Kisspeptin attenuates locomotion by possibly exerting a neuromodulatory role in the mesocorticolimbic dopaminergic pathways (62).
The prefrontal cortex is an area known to express kisspeptin receptor mRNA in humans (52). A recent study in which kisspeptin was administered peripherally to healthy male subjects revealed an increase in the activity of the prefrontal area (as measured by using functional MRI) in response to negative-evoked visual stimuli. Additionally, the investigators indicated a reduction in negative mood as measured with psychometric tests (55). Taken altogether, these findings suggest that the kisspeptin circuitry and signaling responses may have a role in the modulation of mood, anxiety, and reward, by possibly influencing various structures of the limbic and paralimbic networks (53).
Several limbic and paralimbic structures are linked to sexual processing mechanisms in the brain (53). Human studies have indicated that the sexual processing centers in the cingulate and thalamus (cognitive), amygdala and insula (emotional), and putamen and precentral gyrus (motivational) are all involved in the mechanisms leading to the autonomic responses necessary for the induction of sexual behaviors. The kisspeptin neuropeptide and the KP neural circuitry appear to exert an intriguing neuromodulatory role in related neural circuits (55, 67). The exact physiological mechanisms by which kisspeptin modulates sexual behaviors beyond GnRH inductions remain a matter of speculation. However, the kisspeptin signaling pathways, including potential interactions (i.e., anatomically, CN 0), are complex and warrant further study in relation to these behaviors (53).
Conclusions
CN 0 is a highly preserved and functional neuroanatomical structure in most vertebrates, including humans, but one that is not commonly identified and discussed in the medical (e.g., neuroendocrinology, neurology, and neuropsychiatry) and neuroscience communities or in major medical education resources (i.e., neuroanatomy, neuroscience, and clinical textbooks). Recent evidence confirms that CN 0 is not merely a vestigial structure; it is a well-established neuroanatomical structure that is embryologically synergistic with other human CNs and that plays important roles in the development of the GnRH system and the neurophysiology of human reproduction. Its linkage with the hypothalamic kisspeptin neural circuitry yields clinically relevant questions that warrant further investigation of the relationship between CN 0 and the kisspeptin system and the role of neuropsychiatric symptoms and syndromes to which disturbances of this relationship contribute.
1. , , , : Cranial pair 0: the nervus terminalis. Anat Rec (Hoboken) 2019; 302:394–404Crossref, Medline, Google Scholar
2. , : Nervus terminalis (cranial nerve zero) in the adult human. Clin Neuropathol 1990; 9:279–283Medline, Google Scholar
3. : On the organization of olfactory and vomeronasal cortices. Prog Neurobiol 2009; 87:21–30Crossref, Medline, Google Scholar
4. , , : Chapter 9—Cranial nerve 13, in Handbook of Clinical Neurology. Edited by Doty RL. Amsterdam, Elsevier, 2019, pp. 135–144. www.sciencedirect.com/science/article/pii/B9780444638557000095. Accessed December 22, 2021Google Scholar
5. , , , : Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res 1999; 826:220–229Crossref, Medline, Google Scholar
6. : The development of the nervus terminalis in man. J Comp Neurol 1941; 75:39–66Crossref, Google Scholar
7. , , , : Nervus terminalis and nerves to the vomeronasal organ: a study using human fetal specimens. Anat Cell Biol 2019; 52:278–285Crossref, Medline, Google Scholar
8. : Function of gonadotropin-releasing hormone in olfaction. Keio J Med 2001; 50:81–85Crossref, Medline, Google Scholar
9. : Neuroanatomy, cranial nerve 0 (terminal nerve); in StatPearls. Treasure Island, Fla., StatPearls Publishing, 2021. www.ncbi.nlm.nih.gov/books/NBK459159. Accessed April 19, 2021Google Scholar
10. : Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology 2014; 99:33–48Crossref, Medline, Google Scholar
11. , , , : Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92:2744–2750Crossref, Medline, Google Scholar
12. , , : Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol 2013; 784:27–62Crossref, Medline, Google Scholar
13. , , , : The roles of kisspeptin in the mechanism underlying reproductive functions in mammals. J Reprod Dev 2018; 64:469–476Crossref, Medline, Google Scholar
14. , , : The role of kisspeptin in sexual behavior. Semin Reprod Med 2019; 37:84–92Crossref, Medline, Google Scholar
15. , , , : Overview of the history of the cranial nerves: from Galen to the 21st century. Anat Rec (Hoboken) 2019; 302:381–393Crossref, Medline, Google Scholar
16. , , : Cranial nerve nomenclature: historical vignette. World Neurosurg 2019; 128:299–307Crossref, Medline, Google Scholar
17. , : Samuel Thomas von Sömmerring’s contributions on the cranial nerves and vomeronasal organ. Cureus 2018; 10:e2859Medline, Google Scholar
18. , , : On the terminology of cranial nerves. Ann Anat 2011; 193:447–452Crossref, Medline, Google Scholar
19. : The neglected cranial nerve: nervus terminalis (cranial nerve N). Clin Anat 2014; 27:46–53Crossref, Medline, Google Scholar
20. : Terminología Anatómica: new terminology for the new anatomist. Anat Rec 1999; 257:50–53Crossref, Medline, Google Scholar
21. : Terminología Anatómica. dbpedia.org/page/Terminologia_Anatomica. Accessed January 3, 2022Google Scholar
22. : Development of the nervus terminalis: origin and migration. Microsc Res Tech 2004; 65:2–12Crossref, Medline, Google Scholar
23. , : Nervus terminalis derived from the neural crest? a surprising new turn in a century-old debate. Anat Rec B New Anat 2004; 278B:12–13Crossref, Google Scholar
24. , , : Nervous system—ClinicalKey. www.clinicalkey.com/#!/content/book/3-s2.0-B9780323611541000175. Accessed October 5, 2021Google Scholar
25. : Terminal nerve complex. Acta Anat (Basel) 1993; 148:81–95Crossref, Medline, Google Scholar
26. , : Olfactory structures in staged human embryos. Cells Tissues Organs 2004; 178:93–116Crossref, Medline, Google Scholar
27. : Development of olfactory and related structures in staged human embryos. Anat Embryol (Berl) 1980; 161:225–236Crossref, Medline, Google Scholar
28. , : Origin of luteinizing hormone-releasing hormone neurons. Nature 1989; 338:161–164Crossref, Medline, Google Scholar
29. , , , : Ontogeny of GnRH systems. J Reprod Fertil Suppl 1995; 49:147–162Medline, Google Scholar
30. , : Multiple embryonic origins of gonadotropin-releasing hormone (GnRH) immunoreactive neurons. Brain Res Dev Brain Res 1994; 78:279–290Crossref, Medline, Google Scholar
31. , , , : Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Dev Camb Engl 2016; 143:3969–3981Medline, Google Scholar
32. : Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc Res Tech 1999; 44:2–10Crossref, Medline, Google Scholar
33. , , : Gonadotropin-releasing hormone neuronal migration. Semin Reprod Med 2007; 25:305–312Crossref, Medline, Google Scholar
34. : The nervus terminalis. Ann Otol Rhinol Laryngol 1950; 59:414–438Crossref, Medline, Google Scholar
35. : A note on the course and distribution of the nervus terminalis in man. Anat Rec 1915; 9:243–246Crossref, Google Scholar
36. : The peripheral distribution of the nervus terminalis in an infant. J Comp Neurol 1917; 28:349–360Crossref, Google Scholar
37. : The nervus terminalis in adult man. J Comp Neurol 1914; 24:131–135Crossref, Google Scholar
38. , : Angiogenesis in association with the migration of gonadotropic hormone-releasing hormone (GnRH) systems in embryonic mice, early human embryos and in a fetus with Kallmann’s syndrome. Prog Brain Res 2002; 141:59–77Crossref, Medline, Google Scholar
39. , : The terminal nerve (nervus terminalis): structure, function, and evolution: introduction. Ann N Y Acad Sci 1987; 519:ix–xiCrossref, Medline, Google Scholar
40. , , : Ontogenesis of neurons producing luteinizing hormone-releasing hormone (LHRH) in the nervus terminalis of the rat. J Comp Neurol 1985; 238:348–364Crossref, Medline, Google Scholar
41. : Introduction to the anatomy and function of the nervus terminalis. Microsc Res Tech 2004; 65(1–2):1Crossref, Medline, Google Scholar
42. , , , : Role of kisspeptin on hypothalamic-pituitary-gonadal pathology and its effect on reproduction. Cureus 2021; 13:e17600Medline, Google Scholar
43. , , , : KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. JNCI J Natl Cancer Inst 1996; 88:1731–1737Crossref, Google Scholar
44. , , , : Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411:613–617Crossref, Medline, Google Scholar
45. , , , : Nasal placode development, GnRH neuronal migration and Kallmann syndrome. Front Cell Dev Biol 2019; 7:121Crossref, Medline, Google Scholar
46. , , , : Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab 2012; 97:E136–E144Crossref, Medline, Google Scholar
47. , , , : Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100:10972–10976Crossref, Medline, Google Scholar
48. , , , : The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349:1614–1627Crossref, Medline, Google Scholar
49. : GPR54 and kisspeptin in reproduction. Hum Reprod Update 2006; 12:631–639Crossref, Medline, Google Scholar
50. , , , : Can the kisspeptin help us in the understanding of pathology of some neurodegenerative brain diseases? Folia Morphol 2021; 80:756–765Crossref, Medline, Google Scholar
51. , , , : The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276:34631–34636Crossref, Medline, Google Scholar
52. , , , : AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001; 276:28969–28975Crossref, Medline, Google Scholar
53. , : Emerging roles of kisspeptin in sexual and emotional brain processing. Neuroendocrinology 2018; 106:195–202Crossref, Medline, Google Scholar
54. , , , : Kisspeptin modulates gamma-aminobutyric acid levels in the human brain. Psychoneuroendocrinology 2021; 129:105244Crossref, Medline, Google Scholar
55. , , , : Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest 2017; 127:709–719Crossref, Medline, Google Scholar
56. , : Biological significance of kisspeptin-Kiss 1 receptor signaling in the habenula of teleost species. Front Endocrinol 2018; 9:222Crossref, Medline, Google Scholar
57. , , : Habenular kisspeptin modulates fear in the zebrafish. Proc Natl Acad Sci USA 2014; 111:3841–3846Crossref, Medline, Google Scholar
58. , , : Kisspeptin1 modulates odorant-evoked fear response via two serotonin receptor subtypes (5-HT1A and 5-HT2) in zebrafish. J Neurochem 2015; 133:870–878Crossref, Medline, Google Scholar
59. , , : Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul Pept 2013; 180:1–4Crossref, Medline, Google Scholar
60. , , : Effects of kisspeptin on parameters of the HPA axis. Endocrine 2011; 39:220–228Crossref, Medline, Google Scholar
61. , , , : Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol 2009; 21:20–29Crossref, Medline, Google Scholar
62. , , , : Kisspeptin-8 induces anxiety-like behavior and hypolocomotion by activating the HPA axis and increasing GABA release in the nucleus accumbens in rats. Biomedicines 2021; 9:112Crossref, Medline, Google Scholar
63. , , , : Kisspeptin modulates pain sensitivity of CFLP mice. Peptides 2018; 105:21–27Crossref, Medline, Google Scholar
64. , , , : Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol 2010; 22:1101–1112Crossref, Medline, Google Scholar
65. , , , : Locomotor- and reward-enhancing effects of cocaine are differentially regulated by chemogenetic stimulation of gi-signaling in dopaminergic neurons. eNeuro 2018; 5Crossref, Google Scholar
66. , : Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988; 85:5274–5278Crossref, Medline, Google Scholar
67. , , , : Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 1999; 28:1–21Crossref, Medline, Google Scholar



