The Neurobiology of Violence
Abstract
Clinical correlates of violent behavior are known, but the underlying mechanisms are not well understood. This article reviews recent progress in the understanding of such mechanisms involving complex interactions between genes, prenatal and perinatal environmental factors, and rearing conditions. Violent behavior is heterogeneous; that is, impulsive and premeditated violent acts differ in their origins, mechanisms, and management. Recent molecular genetic studies of neurotransmitter regulation are providing new insights into pathophysiology of violent behavior. Functional anatomy of neurotransmitters involved in the regulation of violent behavior is being studied with recently developed brain imaging methods. Increasing evidence indicates commonalities between the neurobiology of violent and suicidal behavior. Progress in the prevention and management of violent behavior depends on studies that address biological factors in their social context. This article updates a previous review.
In the United States, homicide accounts for approximately 20,000 deaths annually.1 A victimization survey estimated that approximately 4.2 violent crimes (assaults, robberies, and rapes) occur annually for each 100 persons older than 12 years.1 Another survey of the general adult population found a 3.7% annual rate of self-reported violent behavior against other persons.2 Thus, violent crime and violent behavior in general cause a major public health problem.
Societal and cultural factors play an important role in the development of violent behavior. However, these environmental factors and their fluctuations elicit different responses in different people. Violent behavior develops as a result of complex interactions between neurobiological and environmental factors. This review will focus primarily on neurobiology. Some of the neurobiological mechanisms of violent behavior are similar or identical to those that operate in suicidal behavior.3
Substance use, personality disorders, major mental disorders, head injuries, and other diagnosable disorders contribute to the level of community violence. However, recent evidence points to neurobiological mechanisms that affect the development and the control of violent behavior but that are not clearly linked to the existing diagnostic categories. These mechanisms involve neurotransmitters that are in part under genetic control; the details are emerging in current research efforts. The main purpose of this article is to update a previous review,4 stressing the neurobiological commonalities between aggressive and suicidal behavior.
DEFINITION AND SUBTYPES OF VIOLENT BEHAVIOR
For the purpose of this review, violent behavior is defined as overt and intentional physically aggressive behavior against another person. Examples include beating, kicking, choking, pushing, grabbing, throwing objects, using a weapon, threatening to use a weapon, and forcing sex. The definition does not include aggression against self. Violent crimes include murder, robbery, assault, and rape. In this review, I will not deal with organized state violence or ethnic warfare.
Violent behavior is heterogeneous in its origins and manifestations. Nevertheless, most violent acts can be classified as impulsive or premeditated, and many perpetrators can be classified as committing predominantly impulsive or predominantly premeditated violent acts. There is increasing evidence for the validity of this distinction. Phenytoin reduced impulsive, but not premeditated, violent acts among prisoners.5 Impulsive violent offenders had lower verbal skills than those committing premeditated violent acts.6 Persons who committed impulsive murders differed from those who committed premeditated ones in their pattern of brain glucose utilization.7 Premeditated violent acts may be either predatory (committed for one's own gain; for example, a robbery) or pathological (committed, for example, by mentally ill people acting on their delusions or hallucinations). The mentally ill, however, may also commit predatory or impulsive violent acts.
CLINICAL CORRELATES OF VIOLENT BEHAVIOR
Substance use disorders (alcohol and drugs) play a major role in violent and suicidal behavior. In the United States, 34% of the risk of violence self-reported by community residents is attributable to substance abuse.2 Urine tests were positive for illicit drugs in 37% to 59% of males arrested for violent crimes in U.S.cities.8 Among Finnish males, 40% of the risk for homicide is attributable to alcoholism.9 There are two types of alcoholism.10 Type 2 accounts for most of the violence associated with alcohol abuse. This type, transmitted from fathers to sons, develops early in life. Type 2 abusers frequently fight under the influence of alcohol and show features of antisocial personality disorder. Personality disorders, particularly the antisocial personality and the borderline personality disorders, are frequently manifested by violent behavior.
A proportion of violent acts occurring in the community are committed by persons diagnosed with major mental disorders such as schizophrenia or mood disorders. Although these disorders carry an elevated risk for violent crime,9,11 only about 4% of the risk for violence self-reported by community residents in the United States is attributable to such disorders.2 However, major mental disorders are frequently comorbid with substance abuse,12 and this comorbidity further elevates the risk for violent behavior.2,13 Patients with major mental disorders who stop taking their medication are at a higher risk for developing violent behavior than those who adhere to the treatment.14 The combination of substance abuse and nonadherence to treatment places patients at a particularly high risk for violence.15 Brain injuries, particularly those affecting the frontal ventromedial areas16 or the temporal lobes, are frequently associated with violent behavior. Dementia, mental retardation, and essentially any other disease or disorder affecting the brain may elicit impulsive violent behavior through cognitive impairment and a failure of inhibitory control.
Rearing environment has a powerful effect on behavior, including violence (Figure 1). At least one-third of victims of child abuse grow up to continue the pattern of inept, neglectful, or abusive rearing as parents.17 Many aspects of the rearing environment are determined by the parents' behavior; that behavior is of course not independent of the parents' genes (a factor not shown in Figure 1). Furthermore, the rearing environment is partly shaped by the child's behavior. Thus, genetic and environmental factors continuously interact in child development.
It is clear that maladaptive behaviors can be learned, but this is not the only mechanism of the intergenerational transfer of violence. Well before any teaching or role modeling takes place, the development of the brain may be affected by various factors in the prenatal and perinatal environment. Fetal exposure to alcohol may contribute to the development of violent behavior.18,19 Early maternal rejection of the child interacts with obstetrical complications to predispose the individual to later (adult) violence.20 Aggressive behavior is heritable,21,22 and these pre- and perinatal events interact with genetic factors. Some of these complex relationships are depicted in Figure 1.
The clinical correlates of suicidal and violent behavior overlap: alcohol and drug abuse, personality disorders, schizophrenia, early life experiences, and various brain disorders predispose to both types of behavior.3
NEUROTRANSMITTERS, THEIR GENETIC CONTROL, AND VIOLENCE
Many neurotransmitters and hormones, including vasopressin,23 steroids, opioids, and other substances, are involved in the modulation of aggressive behavior. Most of the current evidence strongly supports the roles of serotonin and catecholamines.
Serotonin (5-hydroxytryptamine; 5-HT) exerts inhibitory control over impulsive aggression. It is formed in the body by hydroxylation and decarboxylation of the essential amino acid tryptophan. The hydroxylation is the rate-limiting step in 5-HT synthesis; it is catalyzed by tryptophan hydroxylase (TPH). The gene for TPH has been cloned and mapped to the short arm of chromosome 11; two polymorphisms have been identified. The levels of a serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid (CSF) of impulsive offenders were associated with TPH genotype.24 In general, these levels are believed to reflect presynaptic serotonergic activity in the brain. Reduced CSF 5-HIAA levels thus indicate a reduction in central serotonergic activity. At the synaptic level, reuptake is a mechanism for the termination of the action of serotonin. This reuptake is accomplished by a plasma membrane carrier called serotonin transporter (5HTT). The gene for 5HTT has been mapped to chromosome 17; a polymorphism has been described.25 Thus, in summary, serotonin synthesis and activity regulation are under genetic control.
These indices of serotonergic transmission were examined in relation to violent and suicidal behavior. TPH genotype was associated with impulsive-aggressive behavior in male (but not female) patients who had personality disorders26 as well as with suicide attempts in impulsive offenders24 and in nonoffenders.27 Low levels of CSF 5-HIAA have been reported in persons who attempted suicide (particularly by violent means)28 and in persons with personality disorders who showed high levels of lifetime aggressiveness.29 In these subjects, there was an association between aggressiveness and suicidal behavior.29 This trivariate relationship between low levels of CSF 5-HIAA, impulsive aggression, and suicide has been replicated in various other populations.3,4 As mentioned above, type 2 alcoholics frequently exhibit violent behavior; there is a common predisposition underlying the transmission of both alcoholism and violent behavior in families. Type 2 alcoholism is associated with a serotonergic deficit.30–32 An association between low-activity 5HTT promoter genotype and early-onset alcoholism with habitual impulsive violent behavior has been observed.33 Furthermore, a polymorphism of the 5-HT1B receptor gene was linked to alcoholism with aggressive and impulsive behavior.34 A nonhuman primate model confirmed that the serotonergic deficit, assessed by CSF 5-HIAA concentrations, is an antecedent (not a consequence) of alcohol consumption.35
Neuroendocrine challenges offer another method of indirectly studying central serotonergic activity. A single dose of fenfluramine elevates the plasma levels of prolactin. This response is mediated by serotonergic action, and the prolactin elevation thus measures central serotonin activity. Prolactin response is reduced in aggressive subjects; this measure may be more sensitive than the CSF 5-HIAA.36
These measures demonstrated a central serotonergic deficit associated with violence, but they could not locate the deficit to any area within the brain. Such localization has now become possible. Localized reductions of the 5HTT-specific binding were visualized in the living human brain.37 I will return to these results in the section on brain imaging.
Thus, in summary, the association between impulsive violent behavior and central serotonin deficit has been demonstrated by using varied measures of serotonin function. The subjects in these studies were largely diagnosed with personality disorders and alcoholism. The role of serotonergic mechanisms in violent behavior accompanying other disorders is not clear; a small study has failed to detect low levels of CSF 5-HIAA in violent schizophrenic patients.38
Norepinephrine systems also play a role in aggression. Similar to suicidal behavior, aggression is associated with increased noradrenergic activity. Stress-elicited noradrenergic activity is linked to irritable aggression in animals. The evidence for noradrenergic involvement in human aggression is indirect. Plasma levels of epinephrine and norepinephrine were related to experimentally induced hostile behavior in normal subjects.39 Growth hormone response to clonidine, an alpha-2-adrenergic receptor agonist, was related to irritability (but not to assaultiveness) in normal subjects and in patients with personality disorders.40 Furthermore, beta-adrenergic blocking agents have been used clinically to suppress violent behavior in patients with a variety of neuropsychiatric disorders.41
Two enzymes are important in the catabolism of catecholamines, including norepinephrine: monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT). MAO exists in two forms, A and B. Both types are present in the brain; type B is also in the platelets where it can be assayed. Genes for both types are located at the X chromosome. Several lines of evidence link MAO to aggressive behavior. Low MAO activity was found in the platelets of violent offenders.42 In a large kindred, several males showed consistent impulsive violent behavior and mental retardation; each of these males had a point mutation in the MAO-A structural gene that resulted in a complete and selective deficiency of the activity of this enzyme.43 Male “knockout” (genetically altered) mice lacking the MAO-A gene showed aggressive behavior.44 Thus, low levels of MAO activity were associated with impulsive aggression.
COMT activity is governed by a common polymorphism that results in 3- to 4-fold variations in enzymatic activity. Two studies have demonstrated an association between the allele coding for the less active form of the enzyme and violent behavior in schizophrenic and schizoaffective patients.45,46 This association seemed more expressed in males. These small association studies need to be replicated in larger samples of schizophrenic patients and extended to nonschizophrenic subjects. Male (but not female) knockout mice deficient in the COMT gene exhibited aggressive behavior.47 Thus, in summary, high noradrenergic activity and low activity of the enzymes that catabolize catecholamines are associated with aggressive behavior. The roles of dopaminergic mechanisms in relation to alcoholism and personality traits are being studied; the results have not yet provided a generally accepted pattern of association with violence.
In the preceding paragraphs, I discussed various polymorphisms that contribute to the control of neurotransmission. These polymorphisms are genetically transmitted; this is the template function of the gene. This function is not amenable to any changes due to environmental effects except for mutations. However, the transcriptional function that determines the gene expression (i.e., the manufacture of specific proteins) does depend on environmental and social factors.48 Gene expression can be modified, for example, by the rearing environment: CSF 5-HIAA in monkeys varies depending on whether they were reared by their mothers or by peers49 (see Figure 1).
GENDER AND VIOLENCE
Gender is a very robust predictor of violent behavior4 and of suicide.3 In the United States, male suspects account for 85% of arrests for violent crimes.1 Victimization surveys as well as self-reports by adults living in the community2 have generally supported the predominance of males among perpetrators of violence. However, this gender difference is reduced among community residents with major mental disorders and those with substance use disorders,2 and it disappears among hospitalized psychiatric patients.4
The gender difference in aggressiveness develops in preschool years, and it is fully expressed by puberty. The difference is partly due to societal causes, including child-rearing practices. Numerous lines of evidence suggest biological origins of the gender difference. As noted above, the TPH, MAO-A, and COMT genotypes show associations with violent behavior that are largely limited to males. Males are more likely than females to develop alcoholism, particularly type 2 alcoholism that is associated with aggression. Elevated circulating testosterone level may be associated with aggression in young males,50 and perhaps even in young females,51 but these findings have not been consistently replicated; furthermore, it is not clear whether the hormonal level is an antecedent or a consequence of violent behavior.52
BRAIN STRUCTURE AND FUNCTION
The orbitomedial prefrontal cortex is involved in controlling and inhibiting impulsive actions, and lesions to this area may result in disinhibited aggressive4,16 or suicidal behaviors.3 Furthermore, literature on aggression points to a dysfunction of parts of the limbic system, particularly the amygdala and the hippocampus, the two limbic structures within the temporal lobe.53 There is some evidence that the frontal and temporal abnormalities associated with violence may be more expressed in the dominant hemisphere. Until recently, electroencephalography was the main method of studying the function of these systems in humans; this approach was useful in confirming frontotemporal abnormalities in violent individuals (particularly in impulsive violence), but the information was relatively nonspecific in terms of the nature of the dysfunction and its subcortical localization.54,55 More recent imaging methods such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are providing more specific information. Glucose utilization and blood flow measurements using PET showed abnormality in the left temporal lobe and in the frontal lobes of violent psychiatric patients.56 Mentally ill murderers showed selective reductions in prefrontal glucose utilization during a continuous performance task.57 A reanalysis of the data obtained in this study showed that the murderers who had no history of early psychosocial deprivation (e.g., no childhood abuse or family neglect) had lower prefrontal glucose metabolism than murderers with early psychosocial deprivation and a group of normal controls.58 Thus, these results suggest the existence of two paths toward the development of violent behavior: abnormal neurobiology (prefrontal cortical dysfunction), or abnormal rearing environment. Another reanalysis of these data57 determined that the impulsive (but not the predatory) offenders had lower prefrontal glucose metabolism in comparison with the control subjects.7 Thus, prefrontal cortical functioning that controls aggressive behavior was impaired specifically in persons who exhibit impulsive (but not premeditated) violence.
The PET results discussed have revealed brain dysfunctions that were localized, but not specifically linked to any neurotransmitter system. A recent SPECT study reported such specific findings. The 5HTT binding in the midbrain and in the occipital and the frontal mesial cortex of alcoholic impulsive violent offenders was lower than that in healthy control subjects or in nonviolent alcoholics.37 Interestingly, autoradiographic studies of suicide victims' postmortem brain tissue have also revealed serotonin receptor abnormalities in the ventral prefrontal cortex.3 The recent PET and SPECT results discussed above will have to be replicated.
If these studies are correct, what are the origins of the brain dysfunctions they reveal? They may be genetic, or due to noxious environmental influences disturbing prenatal neurodevelopment, or related to perinatal complications, or an interaction among these factors. Some structural changes, such as a reduction of the temporal lobe volume, may be caused by child abuse; this effect could be mediated by chronic elevations of glucocorticoids and catecholamines associated with the stress of being maltreated.59 Similarly, a quantitative EEG study provided preliminary evidence for aberrant cortical development in abused children.60
PREVENTION AND TREATMENT OF VIOLENCE
Improvements in prenatal and perinatal care and prevention of head injuries may reduce the level of violence in the community. Since substance use disorders play such an important role in violence, it seems that recidivistic violent crime could be substantially reduced if the treatment of substance use disorders were routinely available to prisoners. In the United States, the availability is inadequate. Facilities that care for schizophrenic and manic patients need to focus on the diagnosis and treatment of the frequently comorbid substance use disorders. Adherence to treatment must be strongly encouraged and monitored in patients with major mental disorders who have a history of recidivistic violence after they discontinue their medication.
Pharmacological treatment of violent behavior in patients with mental disorders is somewhat similar to the treatment of patients with suicidal ideation. Selective serotonin reuptake inhibitors (SSRIs) that are routinely used to treat depression were also effective against impulsive aggression in patients with personality disorders who were not suffering from major depression.61 Clozapine, an atypical antipsychotic medication, is more effective than other antipsychotics in reducing both aggressive41 and suicidal3 behaviors in schizophrenia. Pharmacological reduction of violent behavior in persons without mental disorders might be possible, but it could raise ethical issues that have not been addressed. Lithium, a medication that appears to reduce suicide risk for bipolar patients,3 also reduced impulsive aggressive behavior in prisoners who were not psychotic;62 both effects could be mediated by an enhancement of serotonergic activity. The administration of an SSRI for 4 weeks to persons without mental disorders has reduced their feelings of hostility.63 Pharmacological treatments of aggression have been recently reviewed in detail elsewhere.41,64,65
FUTURE DIRECTIONS
Phenotypic heterogeneity of violent behaviors is a principal problem of this research area. Efforts at subtyping of violence (e.g., impulsive versus premeditated violence) are in progress, but much remains to be done. Most of the neurobiological tools I discussed have separated, one tool at a time, a group of violent subjects from a group of control subjects. Very rarely has a single tool been used to define subgroups of violent patients; using more than one tool for such grouping is even rarer. But even violent behaviors that are phenotypically identical may be heterogeneous from the standpoint of underlying neurobiology.
A fundamental challenge is to translate the recognition of this heterogeneity into research, prevention, and treatment. This requires using the available tools of molecular genetics, neuropharmacology, brain imaging, and psychopharmacology in the same subjects. In this way, it may, for example, become possible to identify patients with specific serotonergic or noradrenergic deficits that could be corrected pharmacologically, or, in the future, perhaps using gene therapy. Furthermore, such a multidisciplinary approach would enable a search for protective factors that might explain, for example, why some patients with very low CSF 5-HIAA do not become violent. Knowledge of such protective factors would be useful in developing strategies for prevention and treatment.
Testing the efficacy of antiaggressive treatments will require substantial modifications of the classic trial designs that have been used in psychopharmacology for the past three decades. The problems to be addressed include the need for precise definition of violent behavior, the difficulty of measuring outcome because of the relative rarity of violent incidents, bias in the selection of patients for study, inadequate and inappropriate control groups, and inattention to comorbidities and concomitant medications in analyzing results.66
Neurobiological and environmental (e.g., social) factors continuously interact and influence each other. For example, obstetrical complications and maternal rejection interact to raise the propensity for violence, and they may influence each other (e.g., a child with brain dysfunction resulting from obstetrical complications may behave in a way that provokes maternal rejection). To understand the neurobiology of violence, we need to study it in the context of such biosocial interactions.
CONCLUSIONS
The past five years have brought significant progress in our understanding of the neurobiology of violence. The field has been enriched by numerous contributions in the areas of molecular genetics and brain imaging. With deeper understanding of the underlying neurobiology, commonalities between violent and suicidal behaviors can be seen more clearly now.
Up until recently, the assessment of biosocial interactions that result in violent behavior was largely limited to acknowledgments of their existence; we have now progressed to formal statistical demonstrations of these interactions. The “nature or nurture” controversy inherited from Francis Galton has been an underlying philosophical basis of numerous political problems that have plagued the field of violence research during the past decade.67 The recent studies of biosocial interactions have rendered the controversy of nature and nurture moot; nature is nurture.
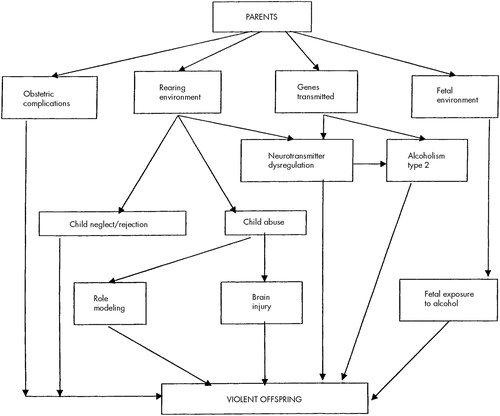
FIGURE 1. Mechanisms for the intergenerational transmission of propensity for violence
1 Sourcebook of Criminal Justice Statistics Online. Washington, DC, U.S. Department of Justice, Bureau of Justice Statistics, 1997Google Scholar
2 Swanson JW: Mental disorder, substance abuse, and community violence: an epidemiological approach, in Violence and Mental Disorder: Developments in Risk Assessment, edited by Monahan J, Steadman HJ. Chicago, University of Chicago Press, 1994, pp 101–136Google Scholar
3 Mann JJ: The neurobiology of suicide. Nat Med 1998; 4:25–30Crossref, Medline, Google Scholar
4 Volavka J: Neurobiology of Violence. Washington, DC, American Psychiatric Press, 1995Google Scholar
5 Barratt ES, Stanford MS, Felthous AR, et al: The effects of phenytoin on impulsive and premeditated aggression: a controlled study. J Clin Psychopharmacol 1997; 17:341–349Crossref, Medline, Google Scholar
6 Barratt ES, Stanford MS, Kent TA, et al: Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biol Psychiatry 1997; 41:1045–1061Google Scholar
7 Raine A, Meloy JR, Bihrle S, et al: Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Sciences and the Law 1998; 16:319–332Crossref, Medline, Google Scholar
8 Maguire K, Pastore AL, Flanagan TJ (eds): Sourcebook of Criminal Justice Statistics 1992. Washington, DC, U.S. Department of Justice, Bureau of Justice Statistics, 1993Google Scholar
9 Eronen M, Hakola P, Tiihonen J: Mental disorders and homicidal behavior in Finland. Arch Gen Psychiatry 1996; 53:497–501Crossref, Medline, Google Scholar
10 Cloninger CR, Bohman M, Sigvardsson S: Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch Gen Psychiatry 1981; 38:861–868Crossref, Medline, Google Scholar
11 Hodgins S, Mednick SA, Brennan PA, et al: Mental disorder and crime: evidence from a Danish birth cohort. Arch Gen Psychiatry 1996; 53:489–496Crossref, Medline, Google Scholar
12 Regier DA, Farmer ME, Rae DS, et al: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990; 264:2511–2518Google Scholar
13 Steadman HJ, Mulvey EP, Monahan J, et al: Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry 1998; 55:393–401Crossref, Medline, Google Scholar
14 Torrey EF: Violent behavior by individuals with serious mental illness. Hospital and Community Psychiatry 1994; 45:653–662Medline, Google Scholar
15 Swartz MS, Swanson JW, Hiday VA, et al: Violence and severe mental illness: the effects of substance abuse and nonadherence to medication. Am J Psychiatry 1998; 155:226–231Medline, Google Scholar
16 Grafman J, Schwab K, Warden D, et al: Frontal lobe injuries, violence, and aggression: a report of the Vietnam head injury study. Neurology 1996; 46:1231–1238Google Scholar
17 Oliver JE: Intergenerational transmission of child abuse: rates, research, and clinical implications. Am J Psychiatry 1993; 150:1315–1324Google Scholar
18 Brown RT, Coles CD, Smith IE, et al: Effects of prenatal alcohol exposure at school age, II: attention and behavior. Neurotoxicology and Teratology 1991; 13:369–376Crossref, Medline, Google Scholar
19 Streissguth AP, Randels SP, Smith DF: A test-retest study of intelligence in patients with fetal alcohol syndrome: implications for care. J Am Acad Child Adolesc Psychiatry 1991; 30:584–587Crossref, Medline, Google Scholar
20 Raine A, Brennan P, Mednick SA: Interaction between birth complications and early maternal rejection in predisposing individuals to adult violence: specificity to serious, early-onset violence. Am J Psychiatry 1997; 154:1265–1271Google Scholar
21 Coccaro EF, Bergeman CS, Kavoussi RJ, et al: Heritability of aggression and irritability: a twin study of the Buss-Durkee aggression scales in adult male subjects. Biol Psychiatry 1997; 41:273–284Crossref, Medline, Google Scholar
22 Bergeman CS, Seroczynski AD: Genetic and environmental influences on aggression and impulsivity, in Neurobiology and Clinical Views on Aggression and Impulsivity, edited by Maes M, Coccaro EF. Chichester, UK, Wiley, 1998, pp 63–80Google Scholar
23 Coccaro EF, Kavoussi RJ, Hauger RL, et al: Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry 1998; 55:708–714Crossref, Medline, Google Scholar
24 Nielsen DA, Goldman D, Virkkunen M, et al: Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry 1994; 51:34–38Crossref, Medline, Google Scholar
25 Collier DA, Stober G, Li T, et al: A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Molecular Psychiatry 1996; 1:453–460Medline, Google Scholar
26 New AS, Gelernter J, Yovell Y, et al: Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: a preliminary study. American Journal of Medical Genetics 1998; 81:13–17Crossref, Medline, Google Scholar
27 Courtet P, Buresi C, Abbar M, et al: Association between the tryptophan hydroxylase gene and suicidal behavior (abstract 146). Presented at the American Psychiatric Association 151st annual meeting, Toronto, Ontario, Canada, May 30–June 4, 1998Google Scholar
28 Åsberg M, Traskman L, Thoren P: 5-HIAA in the cerebrospinal fluid: a biochemical suicide predictor? Arch Gen Psychiatry 1976; 33:1193–1197Google Scholar
29 Brown GL, Goodwin FK, Ballenger JC, et al: Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131–139Crossref, Medline, Google Scholar
30 Virkkunen M, Linnoila M: Serotonin in early-onset, male alcoholics with violent behaviour. Ann Med 1990; 22:327–331Crossref, Medline, Google Scholar
31 LeMarquand D, Pihl RO, Benkelfat C: Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry 1994; 36:326–337Crossref, Medline, Google Scholar
32 Cloninger CR: The psychobiological regulation of social cooperation. Nat Med 1995; 1:623–625Crossref, Medline, Google Scholar
33 Hallikainen T, Saito T, Lachman HM, et al: Association between low activity serotonin transporter promoter genotype with habitual impulsive behavior among antisocial early onset alcoholics. Molecular Psychiatry 1999 (in press)Google Scholar
34 Lappalainen J, Long JC, Eggert M, et al: Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry 1998; 55:989–994Crossref, Medline, Google Scholar
35 Higley JD, Suomi SJ, Linnoila M: A nonhuman primate model of type II excessive alcohol consumption? Part I: low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res 1996; 20:629–642Crossref, Medline, Google Scholar
36 Coccaro EF, Kavoussi RJ, Cooper TB, et al: Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry 1997; 154:1430–1435Google Scholar
37 Tiihonen J, Kuikka JT, Bergstrom KA, et al: Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. European Journal of Nuclear Medicine 1997; 24:1253–1260Google Scholar
38 Kunz M, Sikora J, Krakowski M, et al: Serotonin in violent patients with schizophrenia. Psychiatry Res 1995; 59:161–163Crossref, Medline, Google Scholar
39 Gerra G, Zaimovic A, Avanzini P, et al: Neurotransmitter-neuroendocrine responses to experimentally induced aggression in humans: influence of personality variable. Psychiatry Res 1997; 66:33–43Crossref, Medline, Google Scholar
40 Coccaro EF, Lawrence T, Trestman R, et al: Growth hormone responses to intravenous clonidine challenge correlate with behavioral irritability in psychiatric patients and healthy volunteers. Psychiatry Res 1991; 39:129–139Crossref, Medline, Google Scholar
41 Citrome L, Volavka J: Psychopharmacology of violence, II: beyond the acute episode. Psychiatric Annals 1997; 27:696–703Crossref, Google Scholar
42 Belfrage H, Lidberg L, Oreland L: Platelet monoamine oxidase activity in mentally disordered violent offenders. Acta Psychiatr Scand 1992; 85:218–221Crossref, Medline, Google Scholar
43 Brunner HG, Nelen M, Breakefield XO, et al: Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1993; 262:578–580Crossref, Medline, Google Scholar
44 Cases O, Seif I, Grimsby J, et al: Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 1995; 268:1763–1766Google Scholar
45 Strous RD, Bark N, Parsia SS, et al: Analysis of a functional catechol O-methyltransferase gene polymorphism in schizophrenia: evidence for association with aggressive and antisocial behavior. Psychiatry Res 1997; 69:71–77Crossref, Medline, Google Scholar
46 Lachman HM, Nolan KA, Mohr P, et al: Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am J Psychiatry 1998; 155:835–837Medline, Google Scholar
47 Gogos JA, Morgan M, Luine V, et al: Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95:9991–9996Google Scholar
48 Kandel ER: A new intellectual framework for psychiatry. Am J Psychiatry 1998; 155:457–469Crossref, Medline, Google Scholar
49 Higley JD, Suomi SJ, Linnoila M: A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry 1992; 32:127–145Crossref, Medline, Google Scholar
50 Dabbs JM Jr, Carr TS, Frady RL, et al: Testosterone, crime, and misbehavior among 692 male prison inmates. Personality and Individual Differences 1995; 18:627–633Crossref, Google Scholar
51 Dabbs JM Jr, Hargrove MF: Age, testosterone, and behavior among female prison inmates. Psychosom Med 1997; 59:477–480Crossref, Medline, Google Scholar
52 Susman EJ, Worrall BK, Murowchick E, et al: Experience and neuroendocrine parameters of development: aggressive behavior and competencies, in Aggression and Violence: Genetic, Neurobiological, and Biosocial Perspectives, edited by Stoff DM, Cairns RB. Mahwah, NJ, Lawrence Erlbaum, 1996, pp 267–289Google Scholar
53 Bear D: Neurological perspectives on aggressive behavior. J Neuropsychiatry Clin Neurosci 1991; 3:S3–S8Google Scholar
54 Volavka J: Aggression, electroencephalography, and evoked potentials: a critical review. Neuropsychiatry Neuropsychol Behav Neurol 1990; 3:249–259Google Scholar
55 Convit A, Douyon R, Yates K, et al: Frontotemporal abnormalities and violent behavior, in Aggression and Violence: Genetic, Neurobiological, and Biosocial Perspectives, edited by Stoff DM, Cairns RB. Mahwah, NJ, Lawrence Erlbaum, 1996, pp 169–194Google Scholar
56 Volkow ND, Tancredi L: Neural substrates of violent behaviour: a preliminary study with positron emission tomography. Br J Psychiatry 1987; 151:668–673Crossref, Medline, Google Scholar
57 Raine A, Buchsbaum M, LaCasse L: Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry 1997; 42:495–508Crossref, Medline, Google Scholar
58 Raine A, Phil D, Stoddard J, et al: Prefrontal glucose deficits in murderers lacking psychosocial deprivation. Neuropsychiatry Neuropsychol Behav Neurol 1998; 11:1–7Medline, Google Scholar
59 DeBellis MD, Casey BJ, Clark DB, et al: Anatomical MRI in maltreated children with PTSD (abstract). Biol Psychiatry 1998; 43:16SCrossref, Google Scholar
60 Ito Y, Teicher MH, Glod CA: Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. J Neuropsychiatry Clin Neurosci 1998; 10:298–307Link, Google Scholar
61 Coccaro EF, Kavoussi RJ: Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 1997; 54:1081–1088Google Scholar
62 Sheard MH, Marini JL, Bridges CI, et al: The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976; 133:1409–1413Google Scholar
63 Knutson B, Wolkowitz OM, Cole SW, et al: Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 1998; 155:373–379Crossref, Medline, Google Scholar
64 Citrome L, Volavka J: Psychopharmacology of violence, I: assessment and acute treatment. Psychiatric Annals 1997; 27:691–695Crossref, Google Scholar
65 Citrome L, Volavka J: The efficacy of pharmacological treatments in preventing crime and violence among persons with psychotic disorders, in Violence, Crime, and Mentally Disordered Offenders: Concepts and Methods for Effective Treatment and Prevention, edited by Hodgins S, Muller-Isberner R. New York, Wiley (in press)Google Scholar
66 Volavka J, Citrome L: Atypical antipsychotics in the treatment of the persistently aggressive psychotic patient: methodological concerns. Schizophr Res 1999 (in press)Google Scholar
67 Touchette N: Genetics and crime: It's not over. Till it's over. Journal of NIH Research 1993; 5:32–34Google Scholar



