Personality Disorders Among Medically Refractory Epileptic Patients
Abstract
DSM-III-R personality disorders were assessed in 52 medically refractory epileptic patients. Twenty-one percent of patients met threshold criteria for an Axis II disorder. Dependent and avoidant personality disorders were the most common diagnoses. Epileptic aura was positively correlated with the presence of personality disorders. These results support previous studies that have demonstrated an increased rate of dependency and social isolation in epileptic patients. This increase may be related to disrupted psychosocial functioning as a consequence of having epilepsy, to disrupted neuronal function in central nervous system structures as a consequence of repeated epileptiform discharge or to some combination of the two.
It has been widely reported that patients with epilepsy have increased prevalence of psychopathology compared with healthy control subjects or patients with other chronic disabilities.1–6 Depression is the Axis I psychiatric diagnosis most consistently associated with epilepsy,1–3,6 but other psychiatric diagnoses, such as anxiety and psychosis, also occur among epileptic patients.1,4,5
Whether a characteristic interictal personality profile exists among patients with epilepsy is a controversial issue. Although some authors have found an association between personality traits and epilepsy,7–9 others reported finding no specific personality profiles in epileptic patients when compared to patients with other chronic debilitating disorders.10,11
The lack of consistency in the literature regarding personality abnormalities in patients with epilepsy can be due to different factors. First, authors have used different instruments to measure personality and psychiatric disturbances in the epileptic population. (However, in some cases, results have been inconsistent even when the same instrument was used.8,12) Second, the validity of these instruments has been questioned. Third, the differences between patients with regard to social and psychological attributes and seizure variables might contribute to the dissimilarities in psychiatric disturbances in these patients. Finally, selection bias contributes to the discrepancies because most of the studies have been conducted with patients treated in tertiary medical centers. For example, Stevens13 reported that patients with epilepsy who are treated in medical centers have more psychopathology than those who are treated in private offices. Fiordelli et al.,14 using the Structured Clinical Interview Schedule for DSM-III-R, did not find more Axis I psychiatric disturbances in an outpatient-based clinic compared with age- and sex-matched healthy control individuals.
In patients with a primary psychiatric disorder, comorbidity with DSM-III-R Axis II personality disorders has been associated with poorer response to treatment, lower compliance, and increased risk of suicide attempts (see review15). An increase in personality disorders among patients with epilepsy therefore could have therapeutic and prognostic implications. In addition, it would be of clinical interest to determine whether there is a relationship between epilepsy variables and personality disorders because such a finding might alert the clinician to a risk of possible behavioral complications in a subset of epileptic patients. In the present study, we evaluated the relationship between personality disorders and seizure variables by using a standardized assessment for personality disorders, the Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II).16 We focused on the variables that have been more widely correlated with personality disorders (e.g., type of seizure, laterality, and aura), as well as other variables such as frequency of seizures, duration of illness, and age at illness onset.
METHODS
Subjects with medically refractory seizures who were admitted to the UCLA Seizure Disorder Center for EEG telemetry evaluation as candidates for surgical treatment were selected on the basis of available data for the analysis described in the present study. Informed consent was obtained for all components of the study.
Fifty-seven patients underwent an evaluation with a SCID-II. Psychiatric evaluations were performed by a research psychologist trained in administration of SCID (J.K.), who was blind to seizure type. Five of the patients who underwent evaluation for Axis II disorders were eventually diagnosed with nonepileptic seizures and were excluded from the present study. Data presented herein were obtained from the remaining 52 patients, including 21 men (mean age [±SD], 33.2±11.3 years) and 31 women (mean age 34.8±7.4 years).
For the analysis, personality disorders were divided by clusters, as outlined in DSM-III-R. Cluster A (odd/eccentric) includes schizoid, paranoid, and schizotypal personality disorders. Cluster B (dramatic/emotional) includes borderline, narcissistic, histrionic, and antisocial personality disorders. Cluster C (anxious/fearful) includes avoidant, dependent, compulsive, and passive-aggressive personality disorders. Because of the high prevalence of comorbidity among personality disorders, it has been suggested that these three clusters constitute a more fundamental diagnostic partition of pathology of the personality than do the individual disorders.17
Localization of the epileptogenic region was obtained by a standardized presurgical evaluation that included EEG telemetry, positron emission tomography (PET), magnetic resonance imaging (MRI), and neurocognitive testing.18 All patients underwent both baseline EEG and video-EEG telemetry using scalp and sphenoidal leads. Electroencephalographic criteria included clear localization of at least three typical seizures.
Statistical analysis of nominal variables was performed with chi-square tests, or with Fisher's exact tests when the number of expected values in at least one of the cells was less than 5. Analysis of continuous variables was obtained with unpaired two-tailed t-tests. In addition, logistic regression analysis was performed when appropriate.
RESULTS
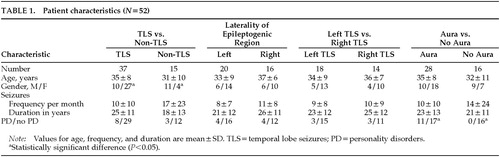
Patient characteristics are shown in Table 1. Thirty-seven patients had temporal lobe seizures (epileptogenic regions: 18 left temporal, 14 right temporal, 5 bilateral temporal). Fifteen patients were diagnosed with other types of seizures (1 absence, 2 bilateral frontal, 2 right frontal, 1 left frontal, 1 left parietal, and 8 generalized seizures of unknown origin). Patient gender and seizure type were found to be associated, the incidence of temporal lobe epilepsy being higher among women than men (Fisher's exact test, P<0.05).
For the analysis, we considered four independent seizure variables: type of seizure (i.e., temporal lobe seizures versus other types); laterality of epileptogenic region in those cases where the region was clearly localized to one side; laterality of temporal lobe seizures; and presence or absence of aura.
Eleven patients (21.15%) were diagnosed with personality disorders among this population. There was no patient with a diagnosis of Cluster A personality disorder. Cluster B was present in 3 patients (5.8%). Cluster C was the most prevalent cluster of all the Axis II diagnoses, occurring in 8 patients (15.4%) and representing 72.7% of patients with any personality disorder diagnosis. When individual personality disorders were considered, we encountered considerable comorbidity within clusters, as previously described:17 4 of the 8 patients with Cluster C personality disorder had more than one diagnosis (3 dependent and avoidant, and 1 obsessive-compulsive and avoidant), and the other 4 patients had only one diagnosis (3 avoidant, 1 dependent). Patients with Cluster B personality disorders consisted of 2 patients with histrionic and 1 with borderline/narcissistic personality disorder. Because of the significant comorbidity, we analyzed the results by grouping the patients by clusters.
Seizure Type
Logistic regression analysis, in which gender and type of seizure were the predictor variables and definite personality disorder was the dependent variable, did not show any statistically significant correlation (P>0.05); therefore, seizure type was not associated with personality disorders when controlling for gender.
Laterality
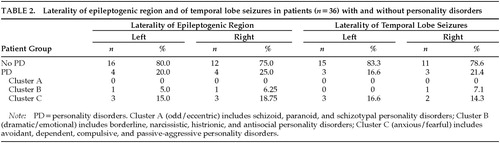
In those patients in whom a lateralized epileptogenic region was identified, laterality of the epileptogenic region was not associated with presence of personality disorder (Table 2; Fisher's exact test, n=36, P=0.51). In addition, since there are reports that left temporal lobe epilepsy is associated with more character pathology,8,19 we performed a separate analysis for patients who had temporal lobe seizures and a lateralized epileptogenic region (Table 2). Laterality of temporal lobe seizures was not associated with presence of personality disorder (Fisher's exact test, n=32, P>0.05).
Aura
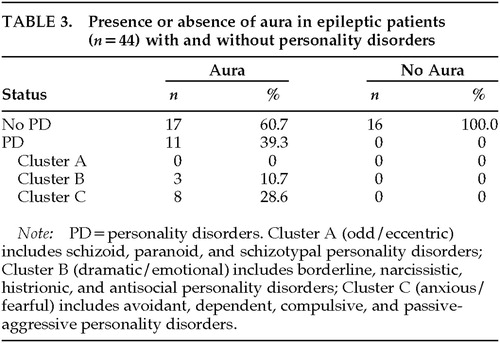
Information regarding presence of aura was obtained from 44 patients. There was no statistically significant association between type of seizures (i.e., temporal lobe seizures versus non–temporal lobe seizures) and presence of aura (χ2 test, n=44, P>0.05), nor between gender and presence of aura (χ2 test, n=44, P>0.05). Therefore, for the analysis of influence of the presence of aura on prevalence of personality disorders, we analyzed the data without adjusting for gender or type of seizure. The presence of aura was found to be positively associated with having a personality disorder (χ2 test, n=44, P<0.005).
No patients without aura suffered from personality disorder, whereas 11 patients with aura (39.3%) had a diagnosis of personality disorder (3 Cluster B and 8 Cluster C; Table 3). The odds ratio (substituting a dummy value of 0.5 for the value 0 in the cell corresponding to patients without aura and with personality disorder) was 20.7.
Other Seizure Variables
We analyzed the effect of other seizure variables—namely, seizure frequency (number of seizure episodes per month), duration of illness, age, and age at onset—on prevalence of personality disorder by using logistic regression. No association was found between these other variables and prevalence of personality disorder (P>0.05). Similarly, no such association was found when we considered only those patients with temporal lobe seizures (P>0.05). In addition, gender was not found to be associated with the presence of personality disorder (χ2 test, n=52, P>0.05).
DISCUSSION
Several studies have found the prevalence of personality disorders in nonpsychiatric populations to range between 5.9% and 11.1%.20–23 Prevalence of personality disorders in the present report is 21.15%, suggesting that epilepsy is associated with an increased prevalence of personality disorders. A limitation of the current study is the lack of matched nonepileptic controls. Matched nonepileptic control subjects and—most important—matched control subjects with other chronic disorders are needed to test more definitely for effects of epilepsy on personality and to assess if an increased prevalence of personality disorders, should it exist in such a study population, is specific to epileptic patients. In addition, it is worth mentioning that the population used for the present study was drawn from medically refractory epileptic patients. It has been shown that severity of epilepsy is associated with decreased psychosocial well-being and increased psychopathology24–26 and that epileptic patients from outpatient clinics have less psychopathology than those studied in inpatient units, who presumably had more severe forms of epilepsy.13,14 Therefore, results obtained in the present report do not necessarily apply to the entire epileptic population.
The most common personality disorders among our patients were Cluster C disorders, specifically dependent and avoidant personality disorders (only one patient met criteria for obsessive personality disorder). Other personality disorders, such as schizoid, paranoid, schizotypal, borderline, antisocial, narcissistic, and histrionic, were not overrepresented in our sample, and we therefore suggest that epilepsy is not a risk factor for the development of these psychopathological entities.
Dependency is one of the most common psychological characteristics of patients with epilepsy.8,9,27 Epilepsy is a disabling disorder that induces a sense of decreased control and self-efficacy, social difficulties, a perception of being stigmatized, and low self-esteem.25,26,28–30 From this point of view, an increase in dependency in epileptic patients might be an expected adaptive reaction to a chronic disease, and therefore not necessarily pathological.
Isolation has also been described as a social problem in epileptic patients (see review31), which may be consistent with our finding of avoidant personality disorder in some of the patients in our population. Therefore, the two personality disorders that are overrepresented in our sample might be accounted for by adaptation or expected reaction to psychosocial disruptions due to epilepsy. Although we expected that these psychosocial problems would be related to severity of epilepsy,31 we did not find a relationship between the duration of seizure disorder or frequency of seizures and these personality disorders. Again, this negative finding might be due to the small number of patients who met criteria for personality disorders in our sample.
Solely on the basis of results from the present study, we cannot rule out the possibility that Cluster C personality disorder in this population has a neuroanatomical basis. Specific lesions of the limbic system increase or decrease social cohesiveness in animals.9 In addition, limbic kindling in rats has been described as decreasing exploration of open arms in the elevated plus-maze test, suggesting increased fearfulness or avoidance.32,33 A higher prevalence of Cluster C disorders (mainly avoidant and dependent) when compared with other types of personality disorders, as outlined in the present report, may thus be due to a neurobiological diathesis, psychosocial consequences induced by epilepsy, or a combination of the two. Specific studies to assess neurobiological abnormalities in patients with seizures, such as PET, might provide valuable insights that address this putative diathesis. For example, it has been reported that decreased metabolic activity in frontal and temporal cortex is positively associated with depression in patients with temporal lobe seizures, thus opening the possibility of a neurobiological substrate for psychopathology in some epileptic patients.34,35
In the present study, the only epilepsy variable that was associated with personality disorders was epileptic aura. Other authors have described an association between auras and psychopathology.6,36,37 Mendez et al.6 reported that patients with auras tended to have a predisposition to depression. Moreover, epileptic patients with personality disorders were found to have more auras, particularly cephalic type, compared with epileptic patients without personality disorders.36 Mendez et al.6 proposed that both the auras and the interictal behavior may result from “epileptiform discharges, seizure-induced kindling of structural and behavioral changes, surround inhibition and hypometabolism, or other neurobiological phenomena,” even though these authors do not discard the possibility that the psychopathology associated with auras may also be the result of negative psychological reactions to unwelcome experiences.
We did not find a relationship between presence of personality disorders and type of seizure or age at onset. Other seizure variables, such as frequency of seizure or duration of illness, also were not associated with the presence of personality disorder, as has been previously described by investigators using other psychological instruments.11,27,38–40 The instrument we used to measure psychopathology (SCID for personality disorders) differed from those used in the reports previously cited. Behavioral characteristics, such as religiosity, hypergraphia, and philosophical interests, are not explored with SCID-III-R for personality disorders, and therefore they were undetected in the population studied in the present report.
It has been reported by some1,9,12,27,41 but not all11,38,40 investigators that patients with temporal lobe seizures (TLS) have more psychopathology than patients with other types of epilepsy. In the present study, we did not find a difference in the rates of personality disorders between patients with temporal lobe seizures and patients with other types of seizures. However, the number of non-TLS patients is relatively small in our sample (only 12), and they represent a variety of seizures disorders. Therefore, conclusive inferences cannot be made.
Hermann et al.38 reported that complex partial seizures have an effect on psychopathology, but only in those patients with an onset of illness during adolescence. These authors found that patients with temporal lobe epilepsy and adolescent age of onset have more psychopathology than patients with either adult or childhood onset of generalized or temporal lobe epilepsy. In the present study, we did not find a statistically significant association between age at onset and prevalence of personality disorders in patients with temporal lobe seizures.
Laterality of epileptogenic region has been correlated with the presence of psychopathology in most8,19,39 but not all12 studies. Using DSM-III-R diagnostic criteria, we have not found a relationship between personality disorders and laterality of the epileptogenic region, but the numbers of subjects may not be large enough to permit definitive conclusions.
In summary, the only personality disorders that were disproportionately represented in our sample were the dependent and avoidant types. The presence of these personality disorders among our patients could be the result of an altered pattern of social relationships due to the psychosocial consequences of living with refractory epilepsy (i.e., disrupted psychosocial functioning as a consequence of sense of loss of control and perception of being stigmatized), or disrupted neuronal functioning due to epileptiform discharges, or some combination of the two. Of all the epilepsy variables, the presence of aura was the only one that was associated with diagnosis of personality disorder. The presence of auras in these refractory patients was correlated with the likelihood of having a personality disorder, and therefore careful exploration by clinicians for the presence of this epilepsy variable and assessment for Axis II pathology may lead to a more comprehensive treatment intervention that takes psychopathology into account.
ACKNOWLEDGMENTS
This work was supported by the National Alliance for Research on Schizophrenia and Depression (grant to Dr. Altshuler). Dr. Lopez-Rodriguez was supported by a Program in Minority Research Training in Psychiatry Fellowship funded by National Institute of Mental Health Grant MH19126. Dr. Engel was supported by National Institute of Neurological Disorders and Stroke Grants NS02808 and NS 33310.
 |
 |
 |
1 Perini GI, Tosin C, Carraro C, et al: Interictal mood and personality disorders in temporal lobe epilepsy and juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry 1996; 61:601–605Crossref, Medline, Google Scholar
2 Blumer D, Montouris G, Hermann B: Psychiatric morbidity in seizure patients on a neurodiagnostic monitoring unit. J Neuropsychiatry Clin Neurosci 1995; 7:445–456Link, Google Scholar
3 Mendez MF, Cummings JL, Benson F: Depression in epilepsy: significance and phenomenology. Arch Neurol 1986; 43:766–770Crossref, Medline, Google Scholar
4 Perini G, Mendius R: Depression and epilepsy in complex partial seizures. J Nerv Ment Dis 1984; 172:287–290Crossref, Medline, Google Scholar
5 Kogeorgos J, Fonagy P, Scott DF: Psychiatric symptoms patterns of chronic epileptics attending a neurological clinic: a controlled investigation. Br J Psychiatry 1982; 140:236–243Crossref, Medline, Google Scholar
6 Mendez MF, Engebrit B, Doss R, et al: The relationship of epileptic auras and psychological attributes. J Neuropsychiatry Clin Neurosci 1996; 8:287–292Link, Google Scholar
7 Waxman SG, Geschwind N: The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry 1975; 32:1580–1586Google Scholar
8 Bear DM, Fedio P: Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol 1977; 34:454–467Crossref, Medline, Google Scholar
9 Bear D, Levin K, Blumer D, et al: Interictal behavior in hospitalised temporal lobe epileptics: relationship to idiopathic psychiatric syndromes. J Neurol Neurosurg Psychiatry 1982; 45:481–488Crossref, Medline, Google Scholar
10 Mungas D: Interictal behavior abnormality in temporal lobe epilepsy. Arch Gen Psychiatry 1982; 39:108–111Crossref, Medline, Google Scholar
11 Whitman S, Hermann BP, Gordon A: Psychopathology in epilepsy: how great is the risk? Biol Psychiatry 1984; 19:213–236Google Scholar
12 Rodin E, Schmaltz S: The Bear-Fedio personality inventory and temporal lobe epilepsy. Neurology 1984; 34:591–596Crossref, Medline, Google Scholar
13 Stevens JR: Interictal manifestations of complex partial seizures, in Advances in Neurology, vol 11, Complex Partial Seizures and Their Treatment, edited by Penry JK, Daly DD. New York, Raven, 1975, pp 85–107Google Scholar
14 Fiordelli E, Beghi E, Bogliun G, et al: Epilepsy and psychiatric disturbances: a cross-sectional study. Br J Psychiatry 1993; 163:446–450Crossref, Medline, Google Scholar
15 Millon T, Kotik-Harper D: The relationship of depression to disorders of personality, in Handbook of Depression, 2nd edition, edited by Beckham EE, Leber WR. New York, Guilford, 1995, pp 107–146Google Scholar
16 Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II, 5/1/86). New York, New York State Psychiatric Institute, Biometric Research Department, 1987Google Scholar
17 Oldham JM, Skodol AE, Kellman HD, et al: Diagnosis of DSM-III-R personality disorders by two structured interviews: patterns of comorbidity. Am J Psychiatry 1992; 149:213–220Crossref, Medline, Google Scholar
18 Engel J Jr: Surgery for seizures. N Engl J Med 1996; 334:647–652Crossref, Medline, Google Scholar
19 Nielsen H, Kristensen O: Personality changes of sphenoidal EEG-foci in temporal lobe epilepsy. Acta Neurol Scandinav 1981; 64:289–300Crossref, Medline, Google Scholar
20 Samuels JF, Nestadt G, Romanoski AJ, et al: DSM-III personality disorders in the community. Am J Psychiatry 1994; 151:1055–1062Google Scholar
21 Maier W, Lichtermann D, Klinger T, et al: Prevalences of personality disorders (DSM-III-R) in the community. J Personal Disord 1992; 6:187–196Crossref, Google Scholar
22 Reich J, Yates W, Nduaguba M: Prevalence of DSM-III personality disorders in the community. Soc Psychiatry Psychiatr Epidemiol 1989; 24:12–16Crossref, Medline, Google Scholar
23 Langner TS, Michael ST: The Stirling County Study of Psychiatric Disorder and Sociocultural Environment. New York, Basic Books, 1963Google Scholar
24 Smith DF, Baker GA, Dewey M, et al: Seizure frequency, patient-perceived seizure severity and the psychosocial consequences on intractable epilepsy. Epilepsy Res 1991; 9:231–241Crossref, Medline, Google Scholar
25 Westbrook LE, Bauman LJ, Shinnar S: Applying stigma theory to epilepsy: a test of a conceptual model. J Pediatric Psychol 1992; 17:633–649Crossref, Medline, Google Scholar
26 Collings JA: Psychosocial well-being and epilepsy: an empirical study. Epilepsia 1990; 31:418–426Crossref, Medline, Google Scholar
27 Hermann BP, Riel P: Interictal personality and behavioral traits in temporal lobe and generalized epilepsy. Cortex 1981; 17:125–128Crossref, Medline, Google Scholar
28 Tedman S, Thornton E, Baker G: Development of a scale to measure core beliefs and perceived self efficacy in adults with epilepsy. Seizure 1995; 4:221–231Crossref, Medline, Google Scholar
29 Collings JA: International differences in psychosocial well-being: a comparative study of adults with epilepsy in three countries. Seizure 1994; 3:183–190Crossref, Medline, Google Scholar
30 Hoare P, Mann H: Self-esteem and behavioral adjustment in children with epilepsy and children with diabetes. J Psychosom Res 1994; 38:859–869Crossref, Medline, Google Scholar
31 Austin JK, deBoer HM: Disruptions in social functioning and services facilitating adjustment for the child and adult, in Epilepsy: A Comprehensive Textbook, edited by Engel J Jr, Pedley TA. Philadelphia, Lippincott-Raven, 1997, pp 2191–2201Google Scholar
32 Kalynchuk LE, Pinel JP, Treit D: Long-term kindling and interictal emotionality in rats: effect of stimulation site. Brain Res 1998; 779:149–157Crossref, Medline, Google Scholar
33 Helfer V, Deransart C, Marescaux C, et al: Amygdala kindling in the rat: anxiogenic-like consequences. Neuroscience 1996; 73:971–978Crossref, Medline, Google Scholar
34 Bromfield EB, Altshuler L, Leiderman DB, et al: Cerebral metabolism and depression in patients with complex partial seizures. Arch Neurol 1992; 49:617–623Crossref, Medline, Google Scholar
35 Victoroff JI, Benson F, Grafton ST, et al: Depression in complex partial seizures: electroencephalographic and cerebral metabolic correlates. Arch Neurol 1994; 51:155–163Crossref, Medline, Google Scholar
36 Mendez MF, Doss RC, Taylor JL, et al: Relationship of seizure variables to personality disorders in epilepsy. J Neuropsychiatry Clin Neurosci 1993; 5:283–286Link, Google Scholar
37 Silberman EK, Sussman N, Skillings G, et al: Aura phenomena and psychopathology: a pilot investigation. Epilepsia 1994; 35:778–784Crossref, Medline, Google Scholar
38 Hermann BP, Schwartz MS, Karnes WE, et al: Psychopathology in epilepsy: relationship of seizure type to age of onset. Epilepsia 1980; 21:15–23Crossref, Medline, Google Scholar
39 Provinciali L, Franciolini B, del Pesce M, et al: Influence of neurological factors on the personality profile of patients with temporal lobe epilepsy. J Epilepsy 1989; 2:239–244Crossref, Google Scholar
40 Swanson SJ, Rao SM, Grafman J, et al: The relationship between seizure subtype and interictal personality. Brain 1995; 118:91–103Crossref, Medline, Google Scholar
41 Rodin EA, Katz M, Lennox K: Difference between patients with temporal lobe seizures and those with other forms of epileptic attacks. Epilepsia 1976; 17:313–320Crossref, Medline, Google Scholar



