Prevalence of Obsessive-Compulsive Disorder in Schizophrenia and Significance of Motor Symptoms
Abstract
To investigate the differences between schizophrenic subjects with and without obsessive-compulsive disorder (OCD), the authors systematically assessed 76 schizophrenic subjects for OCD. Subjects with and without OCD were then compared with regard to motor symptoms, including catatonia, and several measures of psychopathology. Treatment strategies were evaluated retrospectively. The 12 subjects with OCD (15.8%) had more motor symptoms, including catatonia, than non-OCD schizophrenic subjects. Some differences were found with regard to psychopathological symptoms. Treatment strategies also differed in the two groups. The high prevalence of motor symptoms in these subjects supports the hypothesis of a basal ganglia–frontal lobe connection linking OCD with schizophrenia.
The connection between obsessive-compulsive disorder (OCD) and schizophrenia has been of interest to clinicians and researchers since early in this century.1–4 Authors report that between 1% and 16% of patients with OCD developed schizophrenia.5–8 Earlier authors found that OCD occurred in fewer than 1% (N=1,000)2 to 3.5% (N=848)9 of schizophrenic subjects. However, recent studies have reported prevalence rates for OCD in schizophrenia ranging from 7.8%10 to 25%.11 Obsessive-compulsive symptoms have been found in up to 60% of schizophrenic patients.12 The presence of OCD in schizophrenia is reported to predict cognitive impairment, a severe course, and poor outcome.12–16
Despite growing knowledge about OCD and schizophrenia, little is known about the links between them. Traditional authors noted frequent occurrence of stereotypies, mannerisms, negativism, echophenomena, catalepsy, dyskinesias, grimacing, and other catatonic symptoms in schizophrenic patients with OCD2,3,4,17 and also introduced the concept of manneristic catatonia, which described a subtype of schizophrenia with catatonic and obsessive-compulsive symptoms.18 As well, there is one recent report of facial stereotypies in schizophrenic patients with OCD.19
There are also numerous studies on the motor symptoms associated with OCD and extensive reports on obsessive-compulsive features in patients with movement disorders.20–30
This intriguing link between OCD, schizophrenia, and motor symptoms led us to conduct a study in which we identified schizophrenic subjects with and without OCD by means of standardized instruments and then compared the two groups for motor symptoms and for a broad spectrum of clinical parameters. Our hypothesis was that schizophrenic subjects with OCD represent a clinically distinct subset of schizophrenic subjects that can be differentiated on the basis of motor symptoms.
METHODS
Subjects and Diagnosis
Seventy-six subjects were recruited from successive admissions to the General Psychiatry Division, Zentrum für Psychiatrie, University of Bochum, Germany. The hospital is a tertiary care center serving an area of 200,000 people. Admissions cover the spectrum of all psychiatric disorders. All patients are initially admitted to the General Psychiatry Division. After remission of their acute symptoms, they are transferred to specialized inpatient units.
Informed consent was obtained from all subjects after full explanation of the study procedure. The diagnosis of schizophrenia was determined according to DSM-III-R.31 The diagnosis of OCD was determined with the Structured Clinical Interview (SCID) module pertaining to OCD.32 Twelve subjects who fulfilled criteria for OCD were subsequently administered the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS)33 to obtain more detailed information on their OCD. Obsessions were defined as intrusive, persistent, and unwanted ideas, not related to the patient's delusions.10 Compulsions were defined as repetitive behaviors (e.g., hand-washing, checking) or mental acts (e.g., counting) performed in a ritualistic way. The Y-BOCS and the SCID were administered independently by two clinicians. The other scales were administered by five pairs of raters. All raters were experienced clinicians trained in the use of the SCID and the rating scales. Information on the Y-BOCS provided by the patients themselves during the assessment was substantiated by observations of third parties (nurses, treating therapists, family members) and by chart review. Demographic variables and information about clinical course were obtained on admission to the hospital.
Instruments
On admission to the study, patients in both groups were evaluated with a number of rating scales: the Brief Psychiatric Rating Scale (BPRS),34 the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS),35 the Simpson-Angus Rating Scale (SARS) for the assessment of extrapyramidal symptoms,36 the Abnormal Involuntary Movements Scale (AIMS),37 the Hillside Akathisia Scale (HAS),38 and the Catatonia Rating Scale (CRS).39,40 The CRS comprises 16 catatonic motor symptoms and 5 catatonic behaviors. Ratings range from 0 (absent) to 4 (severe). The threshold for diagnosis of catatonia is 4 symptoms rated at least 2 (moderate).
Treatment
Medications were chosen by the attending psychiatrists. Drug information was extracted retrospectively by chart review. Doses of neuroleptics during the index admission, total neuroleptic dose during the week prior to admission, and cumulative lifetime neuroleptic exposure were all converted into chlorpromazine (CPZ) equivalents41 in order to assess differences between the two groups. Treatment with novel antipsychotics, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), benzodiazepines, and anticholinergic drugs was also assessed for both groups.
Statistical Analysis
Statistical analyses, performed by using Statistical Package for the Social Sciences (SPSS) software, included chi-square analysis for categorical data and Fisher's two-tailed exact test when cell sizes were less than 5. Parametric analyses were performed on continuous variables. The unpaired t-test was used for continuous variables, and the Wilcoxon ranked-sum test was used for ordinal data. An analysis of covariance was performed on acute neuroleptic dose, total dose over the week before admission, and cumulative lifetime dose, as well as for treatment with SSRIs, to control for possible medication effect on the motor scales. A hierarchical analysis of covariance was performed on dichotomous treatment variables for the same purpose.
RESULTS
OCD Prevalence and Y-BOCS Scores
The prevalence of OCD in the sample was 15.8% (N=12). Total Y-BOCS scores ranged from 17 to 36. Among these subjects, the most frequent obsessions were of contamination or aggression and somatic obsessions. The most common compulsions were cleaning/washing, checking, repeating, and counting.
Demographic and Illness-Related Information
Twenty-two patients in the total sample were female and 54 were male. The mean age was 35.4 (SD=8.3) years. The mean number of hospitalizations was 5.6. Fifty-eight patients (76%) were single, 11 patients (15%) were employed full time, 19 (25%) were employed part time, and 46 (60%) were unemployed. Thirty-eight patients (50%) received social benefits, and 56 (74%) lived in supervised housing. Mean age of onset of schizophrenia was 20.5 years. No differences were found between the two groups on any of these measures. In 10 of the 12 subjects with OCD, obsessive-compulsive symptoms had been present from childhood and had worsened during adolescence. In the remaining 2 patients, OCD and schizophrenia had begun simultaneously (at a mean age of 20.5 years).
HAS, AIMS, CRS, SARS
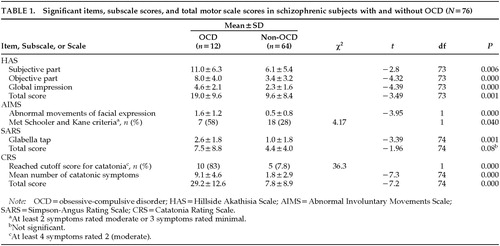
Schizophrenic subjects with OCD had more subjective and objective akathisia and were categorized on the clinical global impression item of the HAS as “markedly akathisic,” in contrast to the non-OCD schizophrenic subjects, who were rated as “borderline akathisic.” Fifty-eight percent of subjects with OCD fulfilled the Schooler and Kane criteria42 for abnormal involuntary movements, as opposed to only 28% of subjects without OCD. Similarly, 83% of subjects with OCD fulfilled CRS criteria for catatonia, as opposed to only 8% of the non-OCD schizophrenic subjects. On the SARS, schizophrenic subjects with OCD were rated as having mild pseudoparkinsonism, but group differences in total SARS scores fell short of significance (Table 1). When all of the scores were controlled for neuroleptic effect, significant main effects for OCD remained robust (main effects for OCD: HAS, F=16.1, P=0.000; AIMS, F=18.3, P=0.000; CRS, F=39.3, P=0.000; SARS, F=3.8, P=0.53).
BPRS, SANS, SAPS
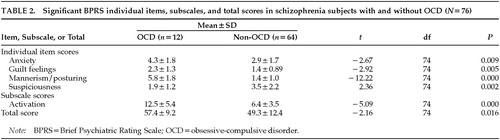
On the BPRS, subjects with OCD had a significantly higher total score than subjects without OCD. They also had significantly more anxiety, guilt feelings, and mannerisms/posturing, but less suspiciousness, than subjects without OCD. On the BPRS subscales, OCD schizophrenic subjects rated higher on the activation subscale than non-OCD schizophrenic subjects (Table 2).
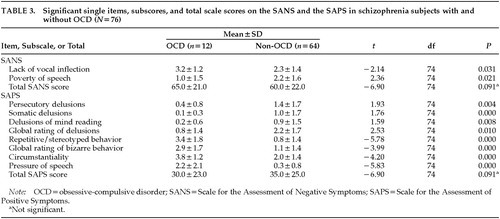
As indicated in Table 3, there were only two items on the SANS on which schizophrenic subjects with OCD significantly differed from those without OCD. No differences were found on the SANS global scale score or on the subscores. On the SAPS, subjects with OCD scored significantly lower on the items and subscales for delusion and hallucinations. They scored significantly higher on the items and subscale for bizarre behavior and on the items “circumstantiality” and “pressure of speech” (Table 3).
Medication
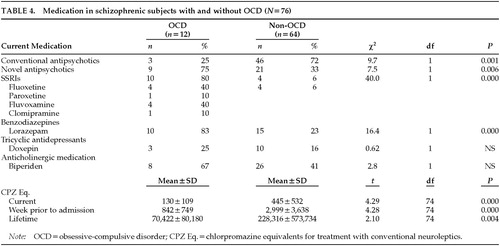
All subjects received more than one type of medication. Table 4 indicates that schizophrenic subjects with OCD had less neuroleptic exposure, as shown by all three measurements of CPZ equivalents, and they were treated more frequently with novel antipsychotics and benzodiazepines than their non-OCD counterparts.
Average dosages of medication other than typical neuroleptics for schizophrenic subjects with and without OCD (OCD-S, Non-OCD-S) included the following: anticholinergics (biperiden), iv 2.5–5 mg/day for acute dystonia and 4–12 mg po/day for parkinsonian-like symptoms (both groups); clozapine, 50–300mg/day (OCD-S) and 150–400 (Non-OCD-S); risperidone, 2–4 mg/day (OCD-S) and 2–6 mg/day (Non-OCD-S); lorazepam, 4–10 mg/day (OCD-S) and 0.5–4 mg (Non-OCD-S); fluoxetine, 40–80 mg/day (OCD-S) and 20 mg/day (Non-OCD-S); paroxetine, 40 mg/day (OCD-S); fluvoxamine, 300 mg/day (OCD-S); clomipramine, 200 mg/day (OCD-S); and doxepine, 11–200 mg/day (OCD-S). All patients received polypharmacy.
DISCUSSION
Prevalence
Based on the prevalence rate of each condition in the community, the expected co-occurrence of schizophrenia with OCD can be calculated as 2%.43,44 The prevalence rate of 15.8% of OCD in our sample is 7 times the chance rate and provides support for earlier reports that a significant subset of schizophrenic patients meets criteria for OCD. Similar rates, ranging between 12.8% and 15%,13,45 have been reported previously. We agree with Eisen et al.10 that a systematic assessment procedure such as the one we used is necessary to reduce the diagnostic confounds in differentiating severe obsessions from psychotic thought disorder and to separate the rituals found in OCD from the coping strategies seen in schizophrenia, which are simple, uniform, and less complex. Furthermore, in clinical practice, prominent positive and/or negative symptoms may mask obsessive-compulsive symptoms. In fact, the relative paucity of literature on OCD in schizophrenia suggests that their co-occurrence is underidentified rather than overdiagnosed.
Motor Symptoms
A major finding in the present study was the high frequency of motor symptoms in the schizophrenic subjects with OCD. This finding supports our central hypothesis. All subjects had been exposed to neuroleptics and other psychotropic medications, but our analysis was corrected for medication exposure and confirmed that the presence of OCD in schizophrenia was strongly associated with motor symptoms. Although the prevalence of extrapyramidal symptoms was not different in the two groups, schizophrenic subjects with OCD had significantly more severe akathisia and more abnormal involuntary movements than their non-OCD counterparts. The pathogenesis of akathisia and abnormal involuntary movements is not clear, and neuroleptic exposure seems to be only one of the factors contributing to their manifestation.46–54 From our findings, it seems likely that schizophrenic patients with OCD are particularly vulnerable to developing both medication-induced neurological motor side effects and medication-unrelated motor symptoms. The high frequency of catatonia in the schizophrenic subjects with OCD confirms clinical observations by authors from the pre-neuroleptic era.3,4
The finding that motor symptoms are a defining feature of schizophrenia with OCD strengthens an emerging connection between OCD, schizophrenia, and motor disorders. There is much direct and indirect evidence pointing to involvement of the frontal lobe and the basal ganglia in the pathophysiology of both schizophrenia and OCD.55–60
OCD has significant associations with a variety of motor symptoms, including stereotypies, grimacing, twitching, catatonia, and tics.21,22,30 There is also evidence in the literature that obsessional slowness, which is characterized by loss of motor fluency, hesitancy of limb movements, speech and gait abnormalities, cogwheel rigidity, complex repetitive movements, and tics and is seen in a subset of patients with OCD, may actually represent a different subtype of OCD.61–63 The clinical description of the symptoms of obsessional slowness overlaps with symptoms of catatonia; however, their relationship has not been explored yet. Conversely, several extrapyramidal motor disorders, most frequently Huntington's disease, tic disorders, distortion dystonia, Tourette's syndrome, and Parkinson's disease,22–30 are known to manifest symptoms of OCD.29 It has been suggested that the links between these disorders and OCD include anatomical and biochemical lesions affecting primarily the basal ganglia and their connections with cerebral regions controlling the frontal lobe and other higher cortical centers.24,64
The involvement of the basal ganglia in catatonia is not fully explored;65,66 however, the symptomatological spectrum of catatonia shows resemblance to the symptoms of extrapyramidal movement disorders.65 There is also evidence from neuroimaging studies pointing to structural abnormalities in the basal ganglia67 and of a basal ganglia regional glucose metabolism asymmetry during catatonic episodes.68 Furthermore, it has been suggested that the efficacy of benzodiazepines in the treatment of catatonia is mediated by potentiation of GABAergic transmission in the basal ganglia.69 Thus, it appears that at least some catatonic symptoms are linked to a dysfunction or abnormality in the basal ganglia. Further studies should identify those catatonic symptoms that are caused by basal ganglia dysfunction.
Positive and Negative Symptoms, General Psychopathology
In the present study, schizophrenic subjects with OCD could not be reliably distinguished from non-OCD schizophrenic subjects on measures of positive and negative symptoms or on general psychopathological symptoms. They do rate higher on the items assessing motor symptoms on all three scales; this is not surprising and substantiates the results on the motor scales.
We found no differences between the two groups on total SAPS and SANS scores. While this is in keeping with the findings of Berman et al.,12 it is in contrast to those of Fenton and McGlashan,13 who reported that schizophrenic subjects with OCD exhibited more negative symptoms.
The higher total score on the BPRS for schizophrenic subjects with OCD is at first suggestive of more general psychopathology, but it is in fact largely due to their high ratings on the abnormal motor items and the anxiety items. We found less suspiciousness in the schizophrenic patients with OCD, which may be a consequence of fewer paranoid delusions.
Diagnostic Relevance
The finding that schizophrenic subjects with OCD exhibit more motor abnormalities than schizophrenic subjects without OCD raises questions of the appropriate classification of this group of patients. One may argue that the comorbid condition may arise as the severe end of the OCD continuum.15 The finding that OCD occurs in several motor disorders has highlighted the linkage between the two and prompted this suggestion.29 Accordingly, OCD without motor abnormalities can be placed at one end of the OCD spectrum, followed by OCD with simple motor symptoms (e.g., tics) and OCD with complex motor disorders (e.g., disorders of the basal ganglia and/or catatonia) at the other end.
Similarly, symptoms involving motion, thought, and perception in OCD may also be placed on a continuum, on which the severe end may merge with schizophrenia.29 For example, symptom progression of motor symptoms will range from tics found in OCD to grimacing and jerky movements seen in schizophrenia. Slow movements in OCD, when exaggerated, become parakinetic and rigid as seen in schizophrenia. Similarly, compulsions and circumstantial movements in OCD may progress into stereotyped movements, rituals, and mannerisms.65 Symptom progression in thought may lead from obsessions to stereotyped thinking and delusions. Thus it is also plausible, in our view, that schizophrenia with OCD may represent the severe end of an OCD spectrum.
Furthermore, there are catamnestic studies in support of the spectrum hypothesis, extending over 1 to 34 years and indicating that childhood OCD may be a precursor of adult psychosis.5–8 Although the reported transition of childhood OCD to adult schizophrenia has been contested,70 it is supported by the fact that the majority of our patients developed OCD in childhood and prior to the onset of schizophrenia. However, to date it is not clear whether schizophrenia with OCD will emerge as a distinct disorder or will ultimately be recognized as a schizophrenia subtype, a comorbid condition, or the severe end of the OCD continuum.15 Alternative explanations of the co-occurrence of the two disorders could be that both are true comorbid conditions or that schizophrenia with OCD may represent a subtype of schizophrenia.
Clinical Implications
Clinicians should assess their schizophrenic patients for OCD if they exhibit tics, other abnormal involuntary movements, catatonic symptoms, or akathisia regardless of neuroleptic treatment. Treatment with SSRIs added to a neuroleptic regimen in these patients is controversial; some authors report it to be of benefit,15,71–74 but others report a possible worsening of psychotic symptoms.75 Although our study was not designed as a treatment study, we found that 8 of the 10 schizophrenic subjects with OCD who were treated with SSRIs showed an improvement of OCD symptoms after an average of 6 weeks, but 2 had no change in symptoms of OCD. Psychotic symptoms worsened in 2 subjects on SSRIs.
Considering the high frequency of motor symptoms in this population, we would suggest that conventional neuroleptics be used with caution. Novel antipsychotics were well tolerated by our schizophrenic subjects with OCD, and in 2 subjects, OCD improved on monotherapy with clozapine. We should note that other authors have reported an induction of OCD with clozapine treatment.72 It was our clinical impression that benzodiazepines were particularly effective in treating both catatonic symptoms and akathisia in these subjects.
Limitations
In general, the lack of universally accepted guidelines to assess OCD in schizophrenia, such as clearly defined criteria to differentiate between obsessions and delusions, has made it difficult to determine prevalence rates. Establishing and using improved criteria is clearly worthwhile because despite the variety of assessment procedures used, all studies have found that OCD in schizophrenia is a clinically relevant condition.
For this study, we assessed obsessive-compulsive symptoms shortly after admission, rather than at several different times during admission. However, we observed that the clinical picture of OCD fluctuated over the hospital course in some patients, and it would therefore be of great interest to systematically assess the longitudinal stability and continuity of OCD in patients with schizophrenia.
To increase the validity of subjects' own statements, clinical assessment of OCD by the raters was augmented by observations from third parties. Our concern was that schizophrenic subjects might suffer impairment in self-observation and might disregard some of their symptoms. Although we did not apply reliability or validity measures to this strategy, it has been successfully used in other studies.40,76
CONCLUSION
We hope that our findings will justify and promote additional studies to further clarify the relationship between OCD, schizophrenia, catatonia, and movement disorders of the extrapyramidal system.
ACKNOWLEDGMENTS
The authors thank D. Surall for statistical assistance. Parts of this study were presented as a poster at the 1998 Annual Meeting of the American Psychiatric Association, Toronto, Ontario, Canada.
 |
 |
 |
 |
1 Bleuler E: Dementia praecox oder Gruppe der Schizophrenien [Dementia praecox or the group of schizophrenias], in Handbuch der Psychiatrie, Spez. Teil, 4 Abt., edited by Aschaffenburg G. Leipzig, Deuticke, 1911Google Scholar
2 Jahrreis W: Über Zwangsvorstellungen im Verlauf der Schizophrenie [On obsessions in schizophrenia]. Archive für Psychiatrie 1926; 77:740–788Crossref, Google Scholar
3 Schneider K: Zwangszustände und Schizophrenie [Compulsive states in schizophrenia]. Archive für Psychiatrie und Nervenkrankeiten 1925; 74:93–107Crossref, Google Scholar
4 Mayer-Gross W: Handbuch der Geisteskrankheiten [Handbook of Mental Illnesses], vol 9, edited by Bumke O. Berlin, Springer, 1932Google Scholar
5 Müller C: Der Übergang von Zwangsneurose in Schizophrenie im Lichte der Katamnese [Transition of compulsive neurosis to schizophrenia as evidenced by catamnesis]. Schweizer Archiv für Neurologie und Psychiatrie 1953; 72:218–225Medline, Google Scholar
6 Pollitt JD: Natural history of obsessional states. British Medical Journal 1957; 1:195–198Crossref, Google Scholar
7 Ingram IM: Obsessional illness in mental hospital patients. Journal of Mental Science 1961; 107:382–402Crossref, Medline, Google Scholar
8 Rosenberg CM: Complications of obsessional neurosis. Br J Psychiatry 1967; 113:823–832Crossref, Medline, Google Scholar
9 Rosen J: The clinical significance of obsessions in schizophrenia. Journal of Mental Science 1957; 103:773–785Crossref, Medline, Google Scholar
10 Eisen JI, Beer DA, Pato MT, et al: Obsessive-compulsive disorder in patients with schizophrenia or schizoaffective disorder. Am J Psychiatry 1997; 154:271–273Crossref, Medline, Google Scholar
11 Berman I, Kalinowski A, Berman SM, et al: Obsessive and compulsive symptoms in chronic schizophrenia. Compr Psychiatry 1995; 35:5–10Google Scholar
12 Berman I, Merson A, Viegner B, et al: Obsessions and compulsions as a distinct cluster of symptoms in schizophrenia: a neuropsychological study. J Nerv Ment Dis 1998; 186:150–156Crossref, Medline, Google Scholar
13 Fenton WS, McGlashan TH: The prognostic significance of obsessive-compulsive symptoms in schizophrenia. Am J Psychiatry 1986; 143:437–441Crossref, Medline, Google Scholar
14 Samuels J, Nestad G, Wolyniec P, et al: Obsessive-compulsive symptoms in schizophrenia (letter). Schizophr Res 1993; 9:139Crossref, Google Scholar
15 Hwang MY, Opler LA: Schizophrenia with obsessive-compulsive features: assessment and treatment. Psychiatric Annals 1994; 24:468–472Crossref, Google Scholar
16 Dowling FG, Pato MT, Pato CN: Comorbidity of obsessive-compulsive and psychotic symptoms: a review. Harv Rev Psychiatry 1995; 3:75–83Crossref, Medline, Google Scholar
17 Huber E, Gross G: Zwangssyndrome bei Schizophrenie [Obsessive-compulsive symptoms in schizophrenia]. Schwerpunktmedizin 1982; 5:12–19Google Scholar
18 Leonhard K: Aufteilung der endogenen Psychosen [Classification of endogenous psychoses], 1 Auflage. Berlin, Akademieverlag, 1957Google Scholar
19 Rogers D, Hymas N: Sporadic facial stereotypies in patients with schizophrenia and compulsive disorders. Adv Neurol 1988; 49:383–394Medline, Google Scholar
20 Arieti S: Interpretation of Schizophrenia. New York, R Brunner, 1955Google Scholar
21 Hermesh H, Hoffnung RA, Aizenberg D, et al: Catatonic signs in severe obsessive compulsive disorder. J Clin Psychiatry 1989; 50:303–305Medline, Google Scholar
22 Kettl PA, Marks IM: Neurological factors in obsessive-compulsive disorder: two case reports and a review of the literature. Br J Psychiatry 1986; 149:315–319Crossref, Medline, Google Scholar
23 Pauls DL, Towbin KE, Lekman JF: Gilles de la Tourette's syndrome and obsessive-compulsive disorder. Arch Gen Psychiatry 1986; 43:1180–1182Google Scholar
24 Pitman RK, Green RC, Jenike MA: Clinical comparison of Tourette's disorder and obsessive-compulsive disorder. Am J Psychiatry 1987; 144:1166–1171Google Scholar
25 Lees AJ: The neurobehavioural abnormalities in Parkinson's disease and their relationship to psychomotor retardation and obsessive-compulsive disorders. Behavioural Neurology 1989; 2:1–11Crossref, Medline, Google Scholar
26 Kienzle JD, Breger RK, Chun RW: Sydenham's MR manifestations in two cases. Am J Neuroradiol 1991; 12:73–76Medline, Google Scholar
27 Cummings JL, Cunningham K: Obsessive-compulsive disorder in Huntington's disease. Biol Psychiatry 1992; 31:263–270Crossref, Medline, Google Scholar
28 Swedo SE, Rapoport JL, Cheslow DL: High prevalence of obsessive-compulsive symptoms in patients with Sydenham's chorea. Am J Psychiatry 1989; 146:246–249Crossref, Medline, Google Scholar
29 Yaryura-Tobias JA, Neziroglu FA: Obsessive-Compulsive Disorder Spectrum: Pathogenesis, Diagnosis, and Treatment. Washington, DC, American Psychiatric Press, 1997Google Scholar
30 DeMarchi N, Morris M, Mennella R, et al: Association of obsessive-compulsive disorder and pathological gambling with Huntington's disease in an Italian pedigree: possible association with Huntington's disease mutation. Acta Psychiatr Scand 1998; 1:62–65Google Scholar
31 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised. Washington, DC, American Psychiatric Association, 1987Google Scholar
32 Spitzer RL, Williams JBW, Gibbon M, et al: Structured Clinical Interview for DSM-III-R (SCID). Washington DC, American Psychiatric Press, 1990Google Scholar
33 Goodman WK, Price LH, Rasmussen SA, et al: The Yale-Brown Obsessive-Compulsive Scale, I: development, use and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Google Scholar
34 Overall JE: The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull 1988; 24:97–99Google Scholar
35 Andreasen NC: The Scales for the Assessment of Negative and Positive Symptoms (SANS and SAPS). Iowa City, IA, University of Iowa, 1983Google Scholar
36 Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
37 US Department of Health, Education and Welfare: Abnormal Involuntary Movements Scale (AIMS), in ECDEU Assessment Manual, edited by Guy W. Rockville, MD, US Department of Health, Education and Welfare, 1976, pp 534–537Google Scholar
38 Fleischhacker WW, Bergmann KJ, Perovich R, et al: The Hillside Akathisia Scale: a new rating instrument for neuroleptic-induced akathisia. J Clin Psychopharmacol 1989; 10:12–21Crossref, Google Scholar
39 Bräunig P, Krüger S, Shugar G, Höffler J, Börner I: The Catatonia Rating Scale I: development, reliability and use. Compr Psychiatry 2000; 41:1–14Crossref, Medline, Google Scholar
40 Bräunig P, Krüger S, Shugar G: Prevalence and clinical significance of catatonic symptoms in mania. Compr Psychiatry 1998; 39:35–47Crossref, Medline, Google Scholar
41 Rey MJ, Schulz P, Costa C, et al: Guidelines for the dosage of neuroleptics, I: chlorpromazine equivalents for orally administered neuroleptics. Int J Clin Psychopharmacol 1989; 4:95–104Crossref, Medline, Google Scholar
42 Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia. Arch Gen Psychiatry 1982; 39: 486–487Google Scholar
43 Foa EB, Kozak MJ: DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry 1995; 143:437–441Google Scholar
44 Karno M, Golding JM, Sorenson SB, et al: The epidemiology of obsessive-compulsive disorder in five U.S. communities. Arch Gen Psychiatry 1988; 45:1094–1099Google Scholar
45 Huber G, Gross G, Schüttler R: Schizophrenie. Berlin, Springer, 1979Google Scholar
46 Kraepelin E: Dementia praecox und Paraphrenie [Dementia praecox and paraphrenia], in Psychiatrie, 8 Auflage. Leipzig, Barth, 1913Google Scholar
47 Fenton WS, Wyatt RJ, McGlashan TH: Risk factors for spontaneous dyskinesia in schizophrenia. Arch Gen Psychiatry 1994; 51: 643–650Google Scholar
48 Bräunig P: Extrapyramidal symptoms in schizophrenia in the preneuroleptic era [trans. from German: EPS bei Schizophrenien bereits in der präneurolpetischen Ära beobachtet], in Differenzierung katatoner und neuroleptikainduzierter Bewegungsstörungen, edited by Bräunig P. Stuttgart, Thieme, 1995, pp 18–24Google Scholar
49 Rogers D: The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry 1985; 147:221–232Crossref, Medline, Google Scholar
50 McCreadie RG, Thara R, Kamath S, et al: Abnormal movements in never-medicated Indian patients with schizophrenia. Br J Psychiatry 1996; 168:221–226Crossref, Medline, Google Scholar
51 Waddington JL, Youssef HA: The lifetime outcome and involuntary movements of schizophrenia never treated with neuroleptic drugs. Br J Psychiatry 1990; 156:106–108Crossref, Medline, Google Scholar
52 Halstead SM, Barnes TRE, Speller JC: Akathisia: prevalence and associated dysphoria in an inpatient population with chronic schizophrenia. Br J Psychiatry 1994; 164:177–183Crossref, Medline, Google Scholar
53 Kurz M, Hummer M, Oberbauer H, et al: Extrapyramidal side-effects of clozapine and haloperidol. Psychopharmacology 1995; 118:52–56Crossref, Medline, Google Scholar
54 Sachdev P, Kane JM: Akathisia and Restless Legs. Cambridge, UK, Cambridge University Press, 1995Google Scholar
55 Breier A, Buchanan RW, Elkashef A, et al: Brain morphology and schizophrenia: a magnetic resonance imaging study of the limbic, prefrontal cortex and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
56 Raine A, Lencz T, Reynolds GP, et al: An evaluation of structural and prefrontal deficits in schizophrenia: MRI and neuropsychological measures. Psychiatry Res: Neuroimaging 1992; 45:123–137Crossref, Medline, Google Scholar
57 Flor-Henry P: Laterality and motility disturbances in psychopathology: a theoretical perspective, in Movement Disorders in Neurology and Neuropsychiatry, edited by Joseph AB, Young RR. Oxford, UK, Blackwell Scientific, 1992, pp 403–419Google Scholar
58 Behcer D, Rapoport JL, Berg MA: Computerized tomography and neuropsychological test measures in adolescents with obsessive-compulsive disorder. Am J Psychiatry 1994; 141:363–369Google Scholar
59 Gray JA: A general model of the limbic system and basal ganglia: applications to schizophrenia and compulsive behaviour of the obsessive type. Rev Neurol 1994; 150:605–613Medline, Google Scholar
60 Abbruzzese M, Bellodi L, Ferri S, et al: Frontal lobe dysfunction in schizophrenia and obsessive-compulsive disorder: a neuropsychological study. Brain Cogn 1995; 27:202–212Crossref, Medline, Google Scholar
61 Rachman S: Primary obsessional slowness. Behav Res Ther 1974; 12:9–18 Crossref, Medline, Google Scholar
62 Hymas N, Lees A, Bolton D, et al: The neurology of obsessional slowness. Brain 1991; 114:2203–2233Google Scholar
63 Ratnasuryia RH, Marks IM, Forshaw DM, et al: Obsessive slowness revisited. Br J Psychiatry 1991; 159:273–274Crossref, Medline, Google Scholar
64 Malloy P: Frontal lobe dysfunction in obsessive-compulsive disorder, in The Frontal Lobes Revisited, edited by Perecman E. New York, IRBN Press, 1987, pp 207–232Google Scholar
65 Rogers D: Motor Disorders in Psychiatry: Towards a Neurological Psychiatry. New York, Wiley, 1992Google Scholar
66 Northoff G, Demisch L, Wenke J, et al: Plasma homovanillic acid concentrations in catatonia. Biol Psychiatry 1996; 39:436–443Crossref, Medline, Google Scholar
67 Bogerts B, Mertz E, Schonfeld-Bausch R: Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42:784–791 Crossref, Medline, Google Scholar
68 Luchins DJ, Metz JT, Marks RC, et al: Basal ganglia regional glucose metabolism asymmetry during a catatonic episode. Biol Psychiatry 1989; 26:725–728Crossref, Medline, Google Scholar
69 Rosebush PI, Mazurek MF: A consideration of the mechanisms by which lorazepam might treat catatonia (letter). J Clin Psychiatry 1991; 52:187–188Google Scholar
70 Hollingworth CE, Tanguay PE, Grossman L, et al: Long-term outcome of obsessive-compulsive disorder in childhood. J Am Acad Child Psychiatry 1980; 19:134–144Crossref, Medline, Google Scholar
71 Berman I, Sapers BL, Chang HJ, et al: Treatment of obsessive-compulsive symptoms in schizophrenic patients with clomipramine. J Clin Psychopharmacol 1995; 15:206–210Crossref, Medline, Google Scholar
72 Poyurovsky M, Hermesh H, Weizman A: Fluvoxamine treatment in clozapine-induced obsessive-compulsive symptoms in schizophrenic patients. Clin Neuropharmacol 1996; 19:305–313Crossref, Medline, Google Scholar
73 Zohar J, Kaplan Z, Benjamin J: Clomipramine treatment of obsessive-compulsive symptomatology in schizophrenic patients. J Clin Psychiatry 1993; 54:385–388Medline, Google Scholar
74 Poyurovsky M, Weizman A: Intravenous clomipramine for a schizophrenic patient with obsessive-compulsive symptoms (letter). Am J Psychiatry 1998; 155:993Crossref, Google Scholar
75 Popli AP, Fuller MA, Jaskiw GE: Sertraline and psychotic symptoms: a case series. Ann Clin Psychiatry 1997; 9:15–17Crossref, Medline, Google Scholar
76 Leckman JF, Riddle MA, Hardin MT, et al: The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989; 28:566–573Crossref, Medline, Google Scholar



