Cognitive Performance in Relation to MRI Temporal Lobe Volume in Schizophrenic Patients and Healthy Control Subjects
Abstract
The aim of this study was to identify whether specific deficits in cognitive processing are present in schizophrenia and whether these are related to the volume of temporal and limbic structures. Twenty-seven schizophrenic outpatients were compared with 19 matched control subjects. Compared with control subjects, patients performed complex tasks disproportionately worse than they performed simple tasks. No group differences were found with regard to temporal and limbic volume. Volume of the parahippocampal gyrus was correlated with cognitive performance. The findings are interpreted as evidence for a dysfunction in the maintenance of task-relevant information and the inhibition of irrelevant information.
Cognitive deficits in schizophrenia may occur across a range of cognitive domains.1,2 The widespread nature of the cognitive problems has raised debate as to whether there is a specific or a general cognitive deficit. The domains often mentioned as differentially compromised include attention,3,4 memory,5,6 and executive functions.7,8 However, most neuropsychological tests involve several cognitive functions, and so it is particularly difficult to identify specific cognitive deficits on the basis of impaired test performance.
In the present study we explored the issue of specific versus general cognitive deficits by using tests of information processing speed. Cognitive speed in schizophrenia may be characterized by a general slowing or, alternatively, by a disproportionate increase in time for certain types of information processing. To investigate the degree to which the slowing of cognitive speed is generalized, two timed tests were used that involve subtasks with increasing complexity. The extra time needed to complete the more demanding subtasks is regarded as a function of the particular task variation. These tests were the Concept Shifting Test (CST)9 and the Stroop Color-Word Test (SCWT).10,11
An advantage of both the SCWT and the CST is that they are relatively short, which reduces the possibility that fatigue might affect performance. If cognitive fatigue did still occur, performance of the second part of the test would be slower than for the first part. The present study examined the possible effects of test duration on the SCWT by comparing speed of performance for the first and the second parts of the test, according to the procedure described by Klein et al.12
The cognitive deficits of patients with schizophrenia have been linked to disturbed frontal lobe functioning13–15 and to changes in the temporal and limbic structures connected to the frontal areas.16–18 Bilder and Szeszko16 argue that a “medial frontolimbic” deficit is central to schizophrenia; such a deficit might be marked by a decrease in the volume of the anterior hippocampus.
The present study investigated whether cognitive processing in schizophrenia is characterized by a general or by a specific slowing and whether this deficit can be related to abnormalities of the temporal and limbic structures.
METHODS
Subjects
Twenty-seven patients with schizophrenia and 19 healthy control subjects were included in the study. The patients were recruited from the social psychiatric service of the Regional Institute for Ambulant Mental Health Care; the psychiatric department of the University Hospital Maastricht; and the ambulatory clinic of the psychiatric hospital Vijverdal, Maastricht, the Netherlands. Patients were diagnosed according to DSM-IV criteria19 by a psychiatrist. The diagnosis was verified according to the Composite International Diagnostic Interview20 by a trained neuropsychologist or psychiatrist. Subject characteristics are shown in Table 1. Patients and control subjects were matched for age, sex, and educational level. Educational level was measured on an 8-point scale, ranging from primary school to higher vocational training and university degree.21 All subjects were right-handed. All patients were outpatients at the time of the assessment, and all received stable doses of antipsychotic medication (mean dose in chlorpromazine equivalents=335 mg; SD=212). Mean total score in the patient group on the Brief Psychiatric Rating Scale22 was 44.4 (SD=10.5).
The control subjects were recruited via newspaper advertisements. None of the control subjects had a history of psychiatric illness or evidence for brain pathology or other major physical pathology, and none of them used psychotropic medication. Exclusion criteria for both groups were a history of CNS illness, head injury that caused unconsciousness for more than 1 hour, any other serious physical illness, and heavy alcohol or drug abuse over the last 12 months. Written informed consent was obtained from all participants.
Neuropsychological Assessment
The following cognitive tests were administered to all subjects: Stroop Color-Word Test,10,11 Concept Shifting Test,9 and three subtests of the Groningen Intelligence Test (GIT).23
The Stroop Color-Word Test involves three subtests that display a hundred stimuli each: color names, colored patches, and color names printed in incongruously colored ink. Subjects are requested to read color-names (card I) or to name colors (card II and III) as fast as possible. Performance on card III is largely determined by the time needed to discard irrelevant but very salient information (verbal), in favor of a less obvious aspect (color naming). During the course of the task, speed of performance is recorded after 4 lines and after 10 lines, which makes it possible to investigate time-on-task effects.
The Concept Shifting Test is a modified version of the Trail Making Test (TMT)24 designed to avoid several methodological problems with the TMT. It consists of three parts. On each test sheet, 16 small circles are grouped in a larger circle. In the smaller circles, the test items (numbers in part A; letters in part B; or both numbers and letters in part C) appear in a fixed random order. Subjects are requested to cross out the items in the correct order (numerical or alphabetical) as fast as possible. Part C requires the subject to alternatively cross out digits and letters, so performance on this part reflects concept-shifting ability.
The three subtasks of the Groningen Intelligence Test were administered to obtain a reliable measure of formal intelligence. The GIT is a test of general intelligence that is used in the Netherlands as much as the Wechsler Adult Intelligence Scale.
Magnetic Resonance Imaging
Images were acquired on a 1.5-tesla MRI scanner (Philips ACS, Eindhoven, the Netherlands). The scanning protocol consisted of a coronal inversion recovery T1-weighted sequence, perpendicular to the long axis of the hippocampus, with a slice thickness of 3 mm (TR/TI/TE 2,100/300/18 ms, matrix 256×179, field of view 23 cm, NEX 2, turbofactor 3, acquisition time 6:53 min). The images were transferred to a standalone SUN workstation and examined with semiautomatic Gyroview software (version 2.1-2 1994; Philips Medical Systems, Eindhoven) containing a manual contouring function.
Regions of interest (ROIs) were outlined on each slice and multiplied by the slice thickness. Volumes of each ROI were derived by summing the relevant consecutive slice volumes. ROIs were the amygdala, hippocampus, parahippocampal gyrus, and temporal lobe. Anatomic guidelines for delineation of the ROIs were established by using whole brain sections and coronal serial sections (from the Laboratory of Pathological Anatomy of the University Hospital Maastricht), an anatomical atlas,25 and previously published guidelines on MRI volume measurements of temporal lobe structures.26,27 In some slices it was difficult to distinguish between the amygdala and the hippocampus. Therefore we decided to divide the amygdala-hippocampus complex into the amygdala–anterior hippocampus and the posterior hippocampus. MRI scans were not available for 2 patients and 2 control subjects.
All measurements were done by the same rater, who was blind to diagnosis and cognitive test results. To assess reliability, scans of 8 subjects were measured twice by the same rater with a period of at least 3 weeks between the measurements. Intraclass correlation coefficients (ICC) were calculated. The reliability of the measurements of the posterior hippocampus was considered insufficient (ICC<0.75), and these data were excluded from further analyses. ICCs of all other structures ranged from 0.85 to 0.98, indicating good test-retest reliability.
Statistical Analysis
All statistical analyses were performed by using SPSS software for Macintosh, version 6.1 (SPSS, Inc., Chicago, IL).
Performance on the SCWT and CST was analyzed by using multivariate analysis of variance (MANOVA). The subject's sex was incorporated as a between-subjects factor in this analysis, as well as in the analysis of the MRI data, because of possible sex differences in schizophrenia.28 To investigate effect of test level, MANOVA with a repeated-measures design was performed for SCWT and CST separately, with group (two levels) as the between-subjects variable and level of test difficulty (three levels) as the within-subjects variable. Contrasts were defined to compare the first with the second level of the task and also the second with the third level.
Time-on-task effects on the SCWT part III were analyzed by calculating mean time to complete a line in the first part of the test and in the second part. Speed ratios were calculated by dividing the mean time needed to complete the first part by the mean time needed to complete the second part; a score of 1 thus means that a subject performed equally well on the first and second halves of the test. To investigate the influence of general ability on cognitive test performance, a post hoc analysis was performed that excluded those subjects with lower than average IQ scores. No analysis of covariance was performed because of preexisting significant differences in IQ level between groups.29
The MRI measures were analyzed by using MANOVA with group and sex as between-subjects factors and laterality as a within-subjects factor. To correct for differences in brain size, height was incorporated as a covariate. Relationships between cognitive performance and MRI measures were computed for each group separately by using Pearson's correlation coefficient. All tests were two-tailed.
RESULTS
MANOVA showed significant main effects for group on the CST and SCWT (F=2.99, df=6,37, P=0.017, Pillai's test). No interaction was found between group and sex. Univariate F-tests revealed that group differences in speed of performance were significant for all measures (F=5.69, df=1,42, P=0.022, for SCWT I; F=11.26, df=1,42, P=0.002, for SCWT II; F=11.18, df=1,42, P=0.002, for SCWT III; F=15.06, df=1,42, P=0.000, for CST A; F=11.09, df=1,42, P=0.002, for CST B; and F=13.57, df=1,42, P=0.001, for CST C). There were no differences in the number of errors made by the two groups on any test. In Figure 1 and Figure 2, completion time is depicted as a function of subtask for SCWT and CST, respectively. The interaction between group and task difficulty was significant for SCWT II vs. SCWT III (F=7.74, df=1,44, P=0.008), but not for SCWT I vs. SCWT II (F=1.99, df=1,44, P=0.166). Similarly, a significant interaction was found between group and task difficulty for CST B vs. CST C (F=7.00, df=1,44, P=0.011), but not for CST A vs. CST B (F=0.21, df=1,44, P=0.646).
Time-on-task effects on the SCWT III were investigated by calculating the speed ratios. Both the schizophrenic group and the control group needed relatively more time to complete the second test part, as indicated by speed ratios less than 1 (mean=0.89, SD=0.14, for the schizophrenic patients; mean=0.91, SD=0.08, for the control subjects). The effect of time on task performance did not differ between the groups, as indicated by the lack of interaction between group and time (F=1.51, df=1,44, P=0.23).
In a post hoc analysis, 8 subjects from the schizophrenia group with lower than average IQ scores (<95) were excluded. The results were similar to those for the whole group, both with regard to the main group effect (F=2.94, df=6,31, P=0.022) and the interaction effect of group and test level, for both SCWT and CST.
MANOVA showed no differences between the two groups in the volume of brain structures (F=0.90, df=6,32, P=0.508; Pillai's test). There was no interaction between group and sex or between group and laterality. In the patient group, the volume of the left parahippocampal gyrus was inversely correlated with performance on SCWT III (R=–0.57, P=0.004). Performance on the other tasks did not correlate with the volume of any of the brain regions investigated. In the control group, no significant correlations were found between brain structure volume and cognitive performance.
DISCUSSION
This study shows that schizophrenic patients are disproportionately slower than control subjects on complex cognitive processing tasks, above a general slowness on simple tasks. This pattern of impairment was also present in patients who had an average or above-average IQ score. Further, the impaired performance of schizophrenic patients on the SCWT was not primarily due to the waning of mental effort. Although a time-on-task effect was present, with performance being typically more rapid on the first part of the test, this was also seen in the control group.
The complex subtasks of the SCWT and the CST involve the maintenance of a certain cognitive set over time and the inhibition of inappropriate responses. The specific slowing in this type of processing is in accordance with recent proposals that the core cognitive deficit in schizophrenia is an impairment in maintaining contextual information, or working memory, and in using that information to inhibit inappropriate responses.15,30 Using a computer simulation model of the Stroop task, Servan-Schreiber and co-workers have demonstrated that when there is a single disturbance to the module that is responsible for representing the context, this produces changes in performance similar to those observed in schizophrenia.30,31 This result suggests that a deficit in the processing of context may underlie various cognitive impairments associated with this disorder. This hypothesis could be tested in future studies by examining whether deficits on tasks that involve maintenance of context can account for deficits in other cognitive domains.
Contrary to our hypothesis, the volume of temporal and limbic structures was not smaller in schizophrenic subjects than in control subjects. The same finding has been reported in other studies.32–34 However, a recent meta-analysis of 18 studies did reveal a bilateral volumetric reduction of the hippocampus, and more tentatively of the amygdala.18 It is possible that the lack of differences in our study is related to characteristics of the patient group, such as relatively mild symptomatology. As yet, however, there is no clear evidence that temporal lobe abnormality is predominantly present in any particular subgroup of patients.17
We found a significant association between the volume of the parahippocampal gyrus and performance on the SCWT III. A significant association between parahippocampal gyrus and cognitive performance has been reported before.35 This result is consistent with a disturbance in the circuitry connecting dorsolateral prefrontal and temporal and limbic areas, a network that is thought to be responsible for the active maintenance of task-relevant information.16 However, in our study only one correlation between cognitive performance and brain structure volume was significant; the possibility of a chance finding thus cannot be excluded, and the finding will need to be replicated.
All patients included in our study used antipsychotic medication, which may have influenced cognitive performance. Recent reviews have suggested, however, that neuroleptics have no marked negative effects on cognition.36,37 Instead, performance may sometimes improve following neuroleptic treatment, possibly because patients receiving medication are more cooperative.37
The present study supports the presence of a disproportionate deficit in the complex processing of information in schizophrenia. This deficit can be explained as a dysfunction in the processing of context.30 It is possible that this deficit is related to a decrease in the volume of the medial temporal areas, but this finding needs to be replicated.
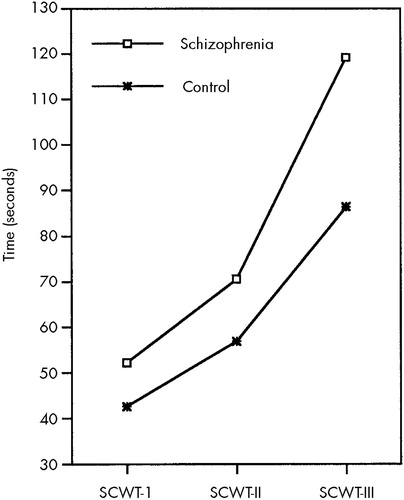
FIGURE 1. Performance on the Stroop Color-Word Test (SCWT) in schizophrenic patients compared with healthy control subjects
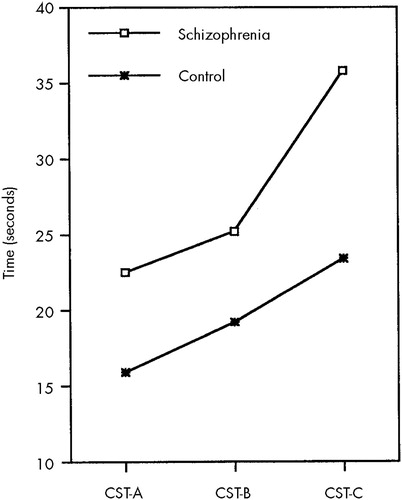
FIGURE 2. Performance on the Concept Shifting Test (CST) in schizophrenic patients compared with healthy control subjects
 |
1 Blanchard JJ, Neale JM: The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry 1994; 151:40–48Google Scholar
2 Braff DL, Heaton R, Kuck J, et al: The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry 1991; 48:891–898Crossref, Medline, Google Scholar
3 Braff DL: Information processing and attention dysfunctions in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
4 Mirsky AF, Yardley SL, Jones BP, et al: Analysis of the attention deficit in schizophrenia: a study of patients and their relatives in Ireland. J Psychiatr Res 1995; 29:23–42Crossref, Medline, Google Scholar
5 McKenna PJ, Tamlyn D, Lund CE, et al: Amnesic syndrome in schizophrenia. Psychol Med 1990; 20:967–972Crossref, Medline, Google Scholar
6 Goldberg TE, Torrey EF, Berman KF, et al: Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatry Res 1994; 55:51–61Crossref, Medline, Google Scholar
7 Morice R, Delahunty A: Frontal/executive impairments in schizophrenia. Schizophr Bull 1996; 22:125–137Crossref, Medline, Google Scholar
8 Evans JJ, Chua SE, McKenna PJ, et al: Assessment of the dysexecutive syndrome in schizophrenia. Psychol Med 1997; 27:635–646Crossref, Medline, Google Scholar
9 Houx PJ, Vreeling FW, Jolles J: Age-associated cognitive decline is related to biological life-events, in Alzheimer's Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies, edited by Iqbal K, McLachlan DRC, Winblad B, et al. Chichester, UK, John Wiley, 1991, pp 353–359Google Scholar
10 Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
11 Houx PJ, Vreeling FW, Jolles J: Stroop interference: aging effects assessed with the Stroop Color-Word Test. Exp Aging Res 1993; 19:209–224Crossref, Medline, Google Scholar
12 Klein M, Ponds RWHM, Houx PJ, et al: Effects of test duration on age-related differences in Stroop Interference. J Clin Exp Neuropsychol 1997; 18:77–82Crossref, Google Scholar
13 Gold JM, Goldberg TE, Weinberger DR: Prefrontal function and schizophrenic symptoms. Neuropsychiatry Neuropsychol Behav Neurol 1992; 5:253–261Google Scholar
14 Frith C: Neuropsychology of schizophrenia, what are the implications of intellectual and experiential abnormalities for the neurobiology of schizophrenia? Br Med Bull 1996; 52:618–626Google Scholar
15 Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Link, Google Scholar
16 Bilder RM, Szeszko PR: Structural neuroimaging and neuropsychological impairments, in Schizophrenia: A Neuropsychological Perspective, edited by Pantelis C, Nelson HE, Barnes TRE. Chichester, UK, John Wiley, 1996, pp 279–298Google Scholar
17 Chua SE, McKenna PJ: Schizophrenia: a brain disease? A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry 1995; 166:563–582Crossref, Medline, Google Scholar
18 Nelson MD, Saykin AJ, Flashman LA, et al: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Arch Gen Psychiatry 1998; 55:433–440Crossref, Medline, Google Scholar
19 American Psychiatric Association: Diagnostic and Statistic Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
20 World Health Organization: Composite International Diagnostic Interview (CIDI); version 1.1, translated by Smeets RMS, Dingemans PMAJ. Geneva, WHO, 1993Google Scholar
21 De Bie SE: Standaardvragen 1987: Voorstellen voor uniformering van vraagstellingen naar achtergrondkenmerken en interviews [Standard questions 1987: proposal for uniformization of questions regarding background variables and interviews], 2nd edition. Leiden, the Netherlands, Leiden University Press, 1987Google Scholar
22 Overall JE, Gorham DE: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
23 Luteijn F, van der Ploeg FAE: Handleiding Groninger Intelligentietest (GIT) [Manual, Groningen Intelligence Test]. Lisse, the Netherlands, Swets and Zeitlinger, 1983Google Scholar
24 Reitan RM: Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
25 Duvernoy H: The human brain: surface, three-dimensional sectional anatomy and MRI. Vienna, Springer-Verlag, 1991Google Scholar
26 Bartzokis G, Mintz J, Marx P, et al: Reliability of in vivo measures of hippocampus and other brain structures using MRI. Magn Reson Imaging 1993; 11:993–1006Google Scholar
27 Watson C, Andermann F, Gloor P, et al: Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 1992; 42:1743–1750Google Scholar
28 Castle DJ, Sham PC, Wessely S, et al: The subtyping of schizophrenia in men and women: a latent class analysis. Psychol Med 1994; 24:41–51Crossref, Medline, Google Scholar
29 Strauss ME, Allred LJ: Measurement of differential cognitive deficits after head injury, in Neurobehavioral Recovery From Head Injury, edited by Levin HS, Grafman J, Eisenberg HM. New York, Oxford University Press, 1987, pp 88–107Google Scholar
30 Servan-Schreiber D, Cohen JD, Steingard S: Schizophrenic deficits in the processing of context: a test of a theoretical model. Arch Gen Psychiatry 1996; 53:1105–1112Google Scholar
31 Cohen JD, Servan-Schreiber D: A theory of dopamine function and its role in cognitive deficits in schizophrenia. Schizophr Bull 1993; 19:85–104Crossref, Medline, Google Scholar
32 Swayze VW, Andreasen NC, Alliger RJ, et al: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
33 DeLisi LE, Tew W, Xie S, et al: A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
34 Young AH, Blackwood DH, Roxborough H, et al: A magnetic resonance imaging study of schizophrenia: brain structure and clinical symptoms. Br J Psychiatry 1991; 158:158–164Crossref, Medline, Google Scholar
35 Nestor PG, Shenton ME, McCarley RW, et al: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849–1855Google Scholar
36 King DJ: The effect of neuroleptics on cognitive and psychomotor function. Br J Psychiatry 1990, 157: 799–811Google Scholar
37 Goldberg TE, Weinberger DR: Effects of neuroleptic medications on the cognition of patients with schizophrenia: a review of recent studies. J Clin Psychiatry 1996; 57(suppl 9):62–65Google Scholar



