The Extended Amygdala and the Dopamine System: Another Piece of the Dopamine Puzzle
Abstract
The dopamine (DA) system has long been associated with the pathophysiology of psychosis. The DA theory of schizophrenia continues to find support in neuroreceptor imaging and ligand-binding studies that show excess DA transmission in patients, as compared to controls. The pathways that regulate the primate DA system, however, have yet to be fully elucidated. The amygdala, including its extended amygdala component, is involved in evaluating the emotional value of sensory stimuli. Since emotionally relevant sensory stimuli are distorted during psychotic episodes, we hypothesize that amygdaloid influences are likely to be significant modulators of the DA system. We reviewed evidence for direct projections from the central extended amygdala to specific subpopulations of DA neurons, and we discuss how these pathways may serve as important conduits of emotionally relevant information that can have immediate and long-term effects on DA regulation.
Psychosis is a devastating feature of many neuropsychiatric disorders, and its pathophysiology remains unknown. While many neurotransmitter systems have been implicated in psychotic illness, dopamine (DA) has a long-standing association with psychosis that remains relevant.1–7 The role of DA in psychosis is currently supported by several ligand-binding studies in humans.8–12 Neuroreceptor imaging shows increased striatal DA turnover in schizophrenic patients after low-dose amphetamine trials that is associated with worsening of positive symptoms in patients.10,12 These findings suggest that exaggerated responses of the DA system may evoke positive symptoms during the acute phase of the illness. The circuitry underlying DA excess in the striatum during acute psychosis remains open to debate. This is due, in part, to the fact that the afferent regulation of DA cells is not fully understood. While the striatum itself is a major afferent of the DA neurons, the amygdala, which plays a key role in emotional function, also sends a projection to the DA system.13–16 This latter pathway has received little attention. In this article, we review the relationship between the amygdala and the DA system in order to shed further light on the modulation of DA under normal and pathologic conditions.
The DA Cells: Recent physiologic studies show that DA cell firing is linked to the presentation of emotionally salient environmental stimuli.17,18 DA neurons are activated in response to primary rewards, novel stimuli, and stimuli that become associated with rewards. DA cells also respond to aversive stimuli, albeit in a more heterogeneous fashion.19,20 Numerous studies show that DA is released in the ventral striatum, amygdala, and prefrontal cortex in response to both rewarding and aversive stimuli,21–29 which indicates that DA functions in signaling aversive as well as rewarding events. Thus, input from limbic structures, such as the amygdala, may enable the DA cells to respond appropriately to emotionally relevant stimuli.
The majority of the brain's DA cells are located in the ventral mesencephalon. Historically, the ventral tegmental area (VTA) has been associated with the “mesolimbic” system, while the main substantia nigra, pars compacta has been considered part of the “nigrostriatal” pathway. More recently, the DA cells have been divided into dorsal and ventral tiers that are based on morphologic, chemical, and connectional properties30–34 (Figure 1). In primates, the dorsal tier encompasses the VTA (A10 neurons), the contiguous pars dorsalis, and the retrorubal field (A8 neurons). This continuous expanse of dorsal tier cells is identified by immunoreactivity to calbindin-D28k, a calcium binding protein. The ventral tier neurons include the main “densocellular” part of the substantia nigra, pars compacta and its vertically oriented cell columns (A9 neurons), which are calbindin-negative.
In primates, the dorsal and ventral tiers are distinguished by differential input/output pathways. The ventral striatum is the main limbic input to the dorsal tier,32–33 which projects back to the ventral striatum, amygdala, and cortex.33,35–38 While cortical inputs to the DA cells are well described in studies performed on rodents,39–40 there is not clear evidence to date of a corticonigral projection in primates (review in Haber and Fudge).41 In contrast to the dorsal tier, the ventral tier is broadly interconnected with the entire striatum through a continuous series of loops33 and has few outputs to the cortex and amygdala. Thus, the dorsal and ventral tiers have differential input and output channels that partially overlap in the ventral striatum.
The Role of the Amygdala: Given evidence suggesting that the DA cells respond to emotionally salient cues, the role of the amygdala in modulating this system is of key importance. The amygdala is a prominent limbic structure that is essential for linking sensory experience to emotional salience.42–44 In humans, amygdaloid activation is readily evoked by emotionally valenced facial expressions.45–49 The dorsal amygdala (corresponding to the region known as the “extended amygdala”) is activated when facial cues switch from neutral to emotional expressions,47 and with changes in emotional intensity of sensory stimuli.50–52 Therefore, the extended amygdala may play an important role in detecting shifts in the relative salience of environmental cues, while the “amygdala proper” functions in the emotional coding of sensory stimuli.
The Amygdala and Psychosis: The possible role of the amygdala in psychosis was identified many years ago through studies on stimulation and electroencephalogram (EEG) abnormalities in schizophrenic patients.53–55 Subsequently, it was reported that direct stimulation of the amygdala in awake humans without schizophrenia elicits a range of complex sensory and emotional states, including fear, anxiety, feeling of familiarity, and complex hallucinations.56,57 Endogenous abnormal amygdaloid stimulation, which can occur in partial complex seizures, has long been associated with the development of “schizophrenia-like” psychoses.58–61 Patients with complex partial seizure and psychosis frequently have brain lesions in or near the amygdala.61–65
The phenomenology of psychotic symptoms underscores the close relationship between the sensory and emotional information that converge in the amygdala. During acute illness, the perception of sensory material deteriorates, along with the interpretation of its emotional relevance. Auditory hallucinations and delusional beliefs are characterized not only by distorted sensory perceptions, but by distinct emotional content or misattribution of emotional meaning to sensory information. Auditory hallucinations, which are experienced as coming from the external world (sensory distortion), are frequently experienced as negatively valenced (emotional content). Consistent with the significant link between emotion and sensory perception, recent studies show that patients with schizophrenia tend to misattribute their own voice to that of another when the voice feedback is slightly distorted or content is derogatory.66
Schizophrenic patients also have difficulty performing tasks that require an evaluation of emotional content of complex sensory stimuli.67–71 Functional imaging abnormalities of the amygdala at rest have been observed in schizophrenic patients performing such tasks. Such findings, however, are variable across studies.72–77 The array of abnormal imaging findings across studies may be due to a number of factors, including heterogeneous symptomatology across samples (varying levels of positive and negative symptoms), the variability in probe stimuli and tasks used across studies, and failure to control for age and gender. The current data suggest that there is not a simple under- or overactivation of the amygdala in schizophrenia, but rather a pattern of inappropriate responses compared to controls, reflecting emotional “coding errors.”
The Amygdala: Anatomy and Pathways: The anatomy of the primate amygdala is consistent with its central role in integrating sensory and emotional information (Fig 2). The basolateral nuclear group (BLNG) is the main nuclear “receiving” group and receives inputs from the temporal cortex, the orbital and medial prefrontal cortex (OMPFC), and the hippocampus.78–81 The temporal cortex mediates higher order auditory and visual processing, while the OMPFC is involved in determining the relative value of reward or punishment.82,83 The hippocampus is vital in the recalling of previously conditioned stimuli.84 These afferent connections provide the necessary elements for evaluating complex sensory data, with respect to emotional salience. Consistent with this anatomic organization, subsets of BLNG neurons are “tuned” to the affective as well as the sensory dimensions of a stimulus. The firing patterns of these neurons are modified as the animal's experience of the stimulus evolves (i.e., if an unfamiliar object becomes familiar or a previously rewarding stimulus becomes aversively conditioned).85 Other major amygdaloid regions include the corticomedial amygdala, which receives olfactory and hippocampal inputs,80,81,86 and the central amygdaloid nucleus (CeN), which has unique connections among other amygdaloid nuclei.
The CeN and Central Extended Amygdala: The CeN stands apart from other amygdaloid regions in that it receives inputs from virtually all the amygdaloid nuclei.87–89 Additionally, the CeN receives information from the “internal milieu” due to its connections with the hypothalamus and autonomic and visceral centers of the brainstem.90–94 CeN neurons are distinguished from the rest of the amygdaloid nuclei because they contain a large array of neuropeptides,95–98 some of which are altered by environmental stress.99,100 These fundamental differences have lead to a recent debate on whether the CeN actually belongs to the amygdala, or whether it is part of another structure known as the “central extended amygdala.”101–103
In the concept of the central extended amygdala, the CeN is part of a cellular continuum that spans the basal forebrain to include another region of the limbic forebrain known as the lateral bed nucleus of the stria terminalis (BSTL)101–104 (Fig. 3). The concept of the central extended amygdala originated in developmental studies that showed that the CeN and BSTL arise as one structure in the fetus and later are divided into rostral (BSTL) and caudal components (CeN) by the fibers of the internal capsule105 (for review).101 Cellular elements remain embedded in the fibers of the internal capsule, forming cell bridges between the two structures. Consistent with a common developmental origin, the BSTL and CeN are symmetrical structures, with respect to their cellular and histochemical organization.97,106–109 In addition to structural similarities, they share similar inputs, including afferents from the basolateral amygdala and hypothalamus.110–112 The medial subregion of the central extended amygdala is unique in that it receives converging inputs from both the BLNG and brainstem centers that are involved in visceral and autonomic function.92,94,113,114 Thus, the medial central extended amygdala is a site where information from the external milieu (via the basolateral amygdala) and internal milieu (via the brainstem/hypothalamus) is combined (see Figure 3C).
The Missing DA Connection: Amygdalonigral Pathway All three amygdaloid subdivisions project to the ventral striatum115 and can, as a result, indirectly influence DA neurons through amygdalo-striato-nigral loops. However, the CeN has a direct input to the DA cells. Older studies conducted on rodents before widespread recognition of the extended amygdala concept indicate that the BSTL, which forms the rostral extended amygdala, also projects to the DA neurons (for review see Fudge and Haber).116–117 These anatomic studies suggested that the entire central extended amygdala might function as an important conduit by which emotionally relevant information influences the DA system. In a series of tract tracing studies, we recently examined how the entire central extended amygdala interacts with specific DA subpopulations in the primate. Injections of retrograde tracers placed within specific DA subpopulations resulted in a near-continuous stream of labeled cells through the central extended amygdala. The BSTL and CeN were labeled together in an “all or none” manner so that labeled cells in the CeN were always accompanied by labeled cells in the BSTL and in cellular bridges throughout the basal forebrain. Conversely, injection sites that failed to label one part of the central extended amygdala also failed to label other regions. These observations support the idea of the central extended amygdala as a unified structure that spans the basal forebrain. The central extended amygdala projects most densely to the dorsal tier neurons, particularly those in the caudal dorsal tier (e.g., “retrorubal” group, A8 neurons). The medial subdivisions of the central extended amygdala and lateral subdivisions that surround the lateral core are the source of dorsal tier inputs (Figure 4). The dorsal part of the ventral tier receives moderate input, but only from the medial subdivision of the central extended amygdala. The ventral cell columns and the pars reticulata receive few, if any, inputs from the central extended amygdala.
In summary, the central extended amygdala influences specific DA subpopulations by two pathways. The medial part of the central extended amygdala, which integrates external stimuli (channeled from the BLNG) and internal cues (from the hypothalamus and brainstem), projects broadly to both the dorsal tier and dorsal portion of the ventral tier. Together, these DA subpopulations project not only to limbic brain regions (the amygdala and ventral striatum) but also to central (cognitive) regions of the striatum and cortex. Converging information from the external and internal environment can, therefore, modulate DA in these diverse brain regions. In contrast, the lateral-most regions of the central extended amygdala project exclusively to the dorsal tier. These lateral extended amygdala regions primarily receive information from external stimuli (via the BLNG and temporal cortex) and are relatively devoid of inputs from the internal milieu. Thus, information about salient stimuli in the animal's external environment is channeled selectively to the dorsal tier neurons, modulating DA in the amygdala, ventral striatum, and cortex.
The Extended Amygdala-Nigral Path: A Direct Link Between the Amygdala and the DA System: The studies above indicate that the central extended amygdala forms a conduit between the BLNG and the DA system. While we have long known of strong inputs from the BLNG to the extended amygdala, the physiology of this connection has only recently been explored. BLNG stimulation results in complex responses in the medial CeN (the caudal portion of central extended amygdala). Detailed analysis indicates that in the medial CeN, neuronal responses depend on both direct glutamatergic inputs from the BLNG and indirect GABAergic input through “intercalated” neurons that lay interposed between the BLNG and CeN. The intercalated neurons receive BLNG inputs and, in turn, inhibit the medial CeN. This arrangement suggests that parallel inhibitory inputs through the intercalated cells “fine tune” excitatory BLNG inputs to the medial CeN neurons. The medial CeN neurons can be activated or suppressed depending on the location, timing, and intensity of BLNG stimulation by this fine tuning mechanism.119–121 Thus, the DA subpopulations that are modulated by the medial CeN are influenced by firing patterns originating in the BLNG, which are further modulated by inputs from intercalated neurons and brainstem and hypothalamic centers. These additional sources of input to the central extended amygdala strongly suggest that it functions as a higher processing center for information that is channeled from the BLNG.
Relevance to Psychosis: The temporal cortex, the OMPFC, and hippocampus are the main glutamatergic projections to the BLNG, and are major sites of structural abnormalities in the schizophrenic brain122–131 (see also review by Arnold (Figure 5).132 Under normal circumstances, these excitatory inputs shape amygdaloid output.133,134 Recent data indicate that such excitatory inputs are in a position to permanently change BLNG responses over time.135–138 Together, these studies indicate that the amygdala is a key site for “emotional learning,” with a high potential for plastic transformation. In schizophrenia, however, these afferent inputs may be structurally impaired, and thus a source of aberrant excitatory signal. Over time, the emotional coding of sensory cues by the amygdala may be compromised due to such faulty inputs. In turn, these “coding errors” can be conveyed to the DA system. Disruption of the DA regulation is hypothesized as a key factor in maintaining psychotic symptoms by reinforcing or sensitizing aberrant brain circuitry.139–140
The BLNG-extended amygdala-nigral path is one way that impaired BLNG signals can dysregulate DA firing patterns over time. Although the physiologic and chemical properties of the extended amygdala-nigral portion of this pathway are far from understood, DA output is affected when afferent signals that originate in the amygdala are impaired.141,142 Furthermore, the DA neurons show complex responses following CeN stimulation, some of which have complex sequences and long durations.143,144 Since the CeN and entire central extended amygdala are characterized by a large number of peptides and transmitters, it is important to delineate how the specific chemical features of this pathway influence DA cell firing in both acute and chronic settings.
CONCLUSION
While the amygdala is almost certainly involved in the emotional coding of environmental stimuli, its precise role in the development of psychotic symptoms remains to be determined. The plasticity of the amygdala and its role in emotional learning suggest that its circuitry is constantly modeled over development. This implies that normal amygdaloid function may be different in childhood and adolescence, the period in which psychosis typically emerges.51 More studies on normal amygdaloid function across the developmental timeline are needed if we are to pinpoint the deterioration of sensory/limbic processing in psychosis. Additionally, the way in which DA is influenced by normal and abnormal amygdaloid signals—both acutely and over time—must be studied further. The peptide and transmitter content of the central extended-amygdala DA pathway also warrant more investigation. Physiologic studies, which detail responses of the DA system to extended amygdala stimulation both acutely and over time, will be useful in understanding the particular function of this path. More detailed elaborations on the link between the amygdala and the DA system will broaden our understanding of the pathophysiology of psychosis and provide another important piece of the DA puzzle.
ACKNOWLEDGMENTS
This was work is supported by MH63291 and the Leonard F. Salzman Award (J.F.).
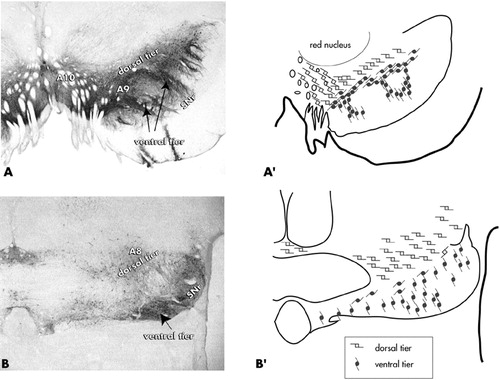
FIGURE 1. Coronal sections showing staining for tyrosine hydroxylase (a marker for dopamine) in the substantia nigra, pars compacta. The “dorsal tier” consists of the A10 neurons (VTA), the contiguous pars dorsalis, and A8 neurons (retrorubal field). The “ventral tier” is comprised of the main group of A9 neurons and their fingerlike column, which penetrate the pars reticulata. Adapted from Haber and Fudge.41
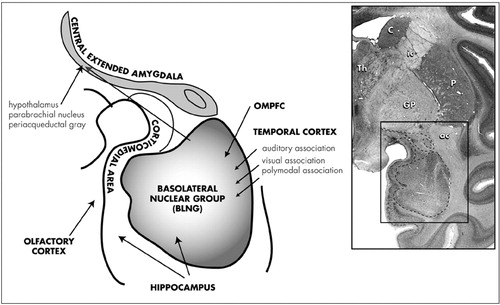
FIGURE 2. Coronal section of nonhuman primate brain at the level of the amygdala stained with cresyl violet (boxed area). Subdivisions of the primate amygdala include: the basolateral nuclear group (BLNG), the corticomedial amygdala, and the central amygdaloid nucleus (the caudal part of the central extended amygdala).
ac=anterior commissure; C=caudate nucleus; GP=globus pallidus; ic=internal capsule; OMPFC=orbital and medial prefrontal cortex; P=putamen; Th=thalamus.
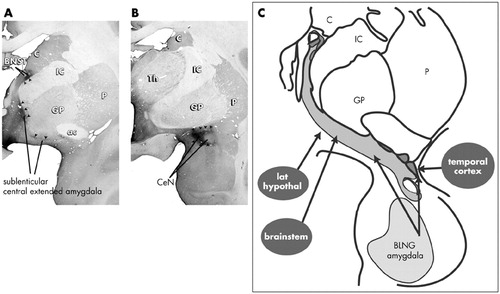
FIGURE 3. Coronal sections through the primate brain at the level of the (A) rostrocentral and (B) caudal central extended amygdala. These levels contain the bed nucleus of the stria terminalis and sublenticular extended amygdala (A), and central nucleus (B). Arrows indicate densely concentrated neurotensin immunoreactivity which marks the medial subdivisions of the central extended amygdala in the primate. (C). The major inputs to the central extended amygdala. Note that the medial central extended amygdala receives afferents from the internal milieu via caudal brainstem and hypothalamus.
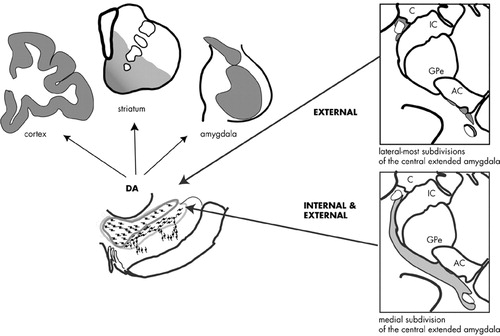
FIGURE 4. Schematic of the central extended amygdala projections to the dopamine subpopulations. Internal and external stimuli are integrated by the medial part of the central extended amygdala (gray). These broad inputs encompass both the dorsal tier + dorsal ventral tier. The output of these combined DA subpopulations includes the ventral (limbic) and central (cognitive) domains of the striatum, the amygdala, and the cortex. The lateral regions of the central extended amygdala are relatively devoid of inputs from the internal milieu and project selectively to the dorsal tier (dark gray). Lateral central cores of the central extended amygdala (white), both in the BSTL and CeN, have few inputs to the DA cells.
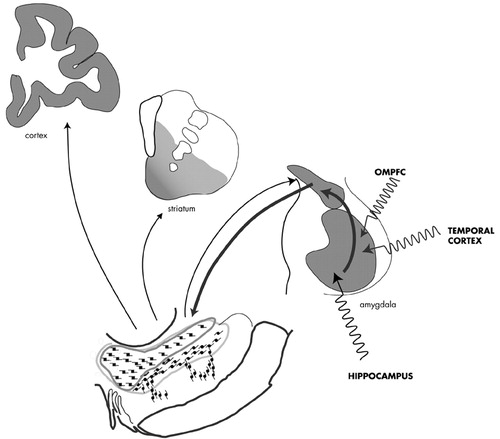
FIGURE 5. The BLNG-extended amygdala-DA pathway: a route for DA dysregulation. The major afferents to the BLNG are structurally abnormal in schizophrenia, placing the BLNG at risk for plastic transformation. The extended amygdala-DA pathway is a route by which impaired amygdaloid function can directly influence DA regulation.
1 Javitt DC, Zukin SR: The role of excitatory amino acids in neuropsychiatric illness. J Neuropsych Clin Neurosci 1990; 2(1):44–52Link, Google Scholar
2 Meltzer HY: Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology 1989; 99 (suppl):S18–27Google Scholar
3 Volk DW, Austin MC, Pierri JN, et al: Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psych 2000; 57(3):237–245Crossref, Medline, Google Scholar
4 Carlsson M, Carlsson A: Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson's disease. Trends Neurosci 1990; 13(7):272–276Crossref, Medline, Google Scholar
5 Matthysse S: Antipsychotic drug actions: a clue to the neuropathology of schizophrenia? Fed Proc 1973; 32:200–205Medline, Google Scholar
6 Creese I, Burt DR, Snyder SH: Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976; 192(4238):481–483Crossref, Medline, Google Scholar
7 Carlsson A: Antipsychotic drugs and catecholamine synapses. J Psych Res 1974; 11:57–64Crossref, Google Scholar
8 Reith J, Benkelfat C, Sherwin A, et al: Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc. Natl Acad Sci U.S.A. 1994; 91(24):11651–11654Google Scholar
9 Abi-Dargham A, Gil R, Krystal J, et al: Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psych 1998; 155(6):761–767Crossref, Medline, Google Scholar
10 Breier A, Su TP, Saunders, et al: Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA 1997; 94(6):2569–2574Crossref, Medline, Google Scholar
11 Hietala J, Syvalahti E, Vuorio K, et al: Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet 1995; 346(8983):1130–1131Crossref, Medline, Google Scholar
12 Laruelle M, Abi-Dargham A, van Dyck CH, et al: Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 1996; 93(17):9235–9240Crossref, Medline, Google Scholar
13 Wallace DM, Magnuson DJ, Gray TS: Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull 1992; 28(3):447–454Crossref, Medline, Google Scholar
14 Bunney BS, Aghajanian GK: The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res 1976; 117:423–435Crossref, Medline, Google Scholar
15 Price JL, Amaral DG: An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1981; 1:1242–1259Crossref, Medline, Google Scholar
16 Gonzales C, Chesselet M-F: Amygdalonigral pathway: an anterograde study in the rat with phaseolus vulgaris leucoagglutinin. J Comp Neurol 1990; 297:182–200Crossref, Medline, Google Scholar
17 Ljungberg T, Apicella P, Schultz W: Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol 1992; 67(1):145–163Crossref, Medline, Google Scholar
18 Mirenowicz J, Schultz W: Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol 1994; 72:1024–1027Crossref, Medline, Google Scholar
19 Guarraci FA, Kapp BS: An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res 1999; 99(2):169–179Crossref, Medline, Google Scholar
20 Mirenowicz J, Schultz W: Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 1996; 379(6564):449–451Crossref, Medline, Google Scholar
21 Abercrombie ED, Keefe KA, DiFrischia DS, et al: Differential effects of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 1989; 52:1655–1658Crossref, Medline, Google Scholar
22 Thierry AM, Tassin JP, Blanc G, et al: Selective activation of mesocortical DA system by stress. Nature 1976; 263(5574):242–244Crossref, Medline, Google Scholar
23 Kalivas PW, Duffy P: Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 1995; 675(1–2):325–328Google Scholar
24 Harmer CJ, Phillips GD: Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience 1999; 90(1):119–130Crossref, Medline, Google Scholar
25 Coco ML, Kuhn CM, Ely TD, et al: Selective activation of mesoamygdaloid dopamine neurons by conditioned stress: attenuation by diazepam. Brain Res 1992; 590(1–2):39–47Google Scholar
26 Inglis FM, Moghaddam B: Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem 1999; 72(3):1088–1094Crossref, Medline, Google Scholar
27 Richardson NR, Gratton A: Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J Neurosci 1996; 16:8160–8169Crossref, Medline, Google Scholar
28 Richardson NR, Gratton A: Changes in the medial prefrontal cortical dopamine levels associated with response-contingent food reward: an electrochemical study in rat. J Neurosci 1998; 18(21):9130–9138Crossref, Medline, Google Scholar
29 Blackburn JR, Phillips AG, Fibiger HC: Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci 1987; 101(3):352–360Crossref, Medline, Google Scholar
30 Lavoie B, Parent A: Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport 1991; 2 (10):601–604Google Scholar
31 Hirsch EC, Mouatt A, Thomasset M, et al: Expression of calbindin D28K–like immunoreactivity in catecholaminergic cell groups of the human midbrain: normal distrubution and distrubution in Parkinson's disease. Neurodegeneration 1992; 1:83–93Google Scholar
32 Lynd-Balta E, Haber SN: Primate striatonigral projections: a comparison of the sensorimotor-related striatum and the ventral striatum. J Comp Neurol 1994; 343:1–17Crossref, Medline, Google Scholar
33 Haber SN, Fudge JL, McFarland N: Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20(6):2369–2382Crossref, Medline, Google Scholar
34 McRitchie DA, Halliday GM: Calbindin D28K-containing neurons are restricted to the medial substantia nigra in humans. Neuroscience 1995; 65:87–91Crossref, Medline, Google Scholar
35 Gaspar P, Stepneiwska I, Kaas JH: Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol 1992; 325:1–21Crossref, Medline, Google Scholar
36 Porrino LJ, Goldman-Rakic PS: Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol 1982; 205:63–76Crossref, Medline, Google Scholar
37 Fudge JL, Haber SN: Dopamine innervation of the amygdala in primates. Soc Neuro Abst 1999; 25(2):2172Google Scholar
38 Williams SM, Goldman-Rakic PS: Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex 1998; 8(4):321–345Crossref, Medline, Google Scholar
39 Phillipson OT: Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol 1979; 187:117–144Crossref, Medline, Google Scholar
40 Sesack SR, Pickel VM: Prefrontal cortical efferents in the rat synapse on unlabled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol 1992; 320:145–160Crossref, Medline, Google Scholar
41 Haber SN, Fudge J: The interface between dopamine neurons and the amygdala: Implications for schizophrenia. Schizophr Bull 1997; 23 (3):471–482Google Scholar
42 Weiskrantz L: Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 1956; 49:381–391Crossref, Medline, Google Scholar
43 Blanchard DC, Blanchard RJ: Innate and conditioned reaction to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 1972; 81:281–290Crossref, Medline, Google Scholar
44 Aggleton JP: The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 1993; 16:328–333Crossref, Medline, Google Scholar
45 Fried I, MacDonald KA, Wilson CL: Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 1997; 18(5):753–765Crossref, Medline, Google Scholar
46 Morris JS, Friston KJ, Buchel C, et al: A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 1998; 121(Pt 1):47–57Google Scholar
47 Whalen PJ, Rauch SL, Etcoff NL, et al: Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18:411–418Crossref, Medline, Google Scholar
48 Breiter HC, Etcoff NL, Whalen PJ, et al: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17(5):875–887Crossref, Medline, Google Scholar
49 Critchley H, Daly E, Phillips M, et al: Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 2000; 9(2):93–105Crossref, Medline, Google Scholar
50 Taylor SF, Liberzon I, Koeppe RA: The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia 2000; 38(10):1415–1425Crossref, Medline, Google Scholar
51 Thomas KM, Drevets WC, Whalen PJ, et al: Amygdala response to facial expressions in children and adults. Biol Psychiatry 2001; 49(4):309–316Crossref, Medline, Google Scholar
52 Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P: Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001; 30(2):619–639Crossref, Medline, Google Scholar
53 Stevens JR: An anatomy of schizophrenia? Arch Gen Psychiatry 1973; 29:177–189Crossref, Medline, Google Scholar
54 Heath RG, Monroe RR, Mickle WA: Stimulation of the amygdaloid nucleus in a schizophrenic patient. Am J Psychol 1954; 111:862–863Crossref, Google Scholar
55 Torrey EF, Peterson MR: Schizophrenia and the limbic system. Lancet 1974; 2:942–946Crossref, Medline, Google Scholar
56 Halgren E, Walter RD, Cherlow DG, et al: Mental phenomena evoked by electrical stimulation of the human hippocampal formation and amygdala. Brain 1978; 101:83–117Crossref, Medline, Google Scholar
57 Gloor P, Oliver A, Quesney LF, et al: The role of the limbic system in experimental phenomena of temporal lobe epilepsy. Ann Neurol 1982; 12:129–144Crossref, Medline, Google Scholar
58 Flor-Henry P: Schizophrenic-like reactions and affective psychoses associated with temporal lobe epilepsy: etiological factors. Am J Psychiatry 1969; 126:400–404Crossref, Medline, Google Scholar
59 Stevens JR: Interictal clinical manifestations of complex partial seizures. Adv Neurol 1975; 11:85–112Medline, Google Scholar
60 Mendez MF, Grau R, Doss RC, et al: Schizophrenia in epilepsy: Seizure and psychosis variables. Neurology 1993; 43:1073–1077Crossref, Medline, Google Scholar
61 Roberts GW, Done DJ, Bruton C, Crow TJ: A “mock up” of schizophrenia: temporal lobe epilepsy and schizophrenia-like psychosis. Biol Psychiatry 1990; 28:127–143Crossref, Medline, Google Scholar
62 Falconer MA: Reversibility by temporal-lobe resection of the behavioral abnormalites of temporal-lobe epilepsy. N Engl J Med 1973; 289:451–455Crossref, Medline, Google Scholar
63 Wolf HK, Campos MG, Zentner J, et al: Surgical pathology of temporal lobe epilepsy. Experience with 216 cases. J Neuropath Exp Neurol 1993; 52:499–506Crossref, Medline, Google Scholar
64 Savard G, Andermann F, Olivier A, et al: Postical psychosis after partial complex seizures: a multiple case study. Epilepsia 1991; 32:225–231Crossref, Medline, Google Scholar
65 Hudson LP, Munoz DG, Miller L, et al: Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol 1993; 33(6):622–631Crossref, Medline, Google Scholar
66 Johns LC, Rossell S, Frith C, et al: Verbal self-monitoring and auditory verbal hallucinations in patients with schizophrenia. Psychol Med 2001; 31(4):705–715Crossref, Medline, Google Scholar
67 Heimberg C, Gur RE, Erwin RJ, et al: Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psych Res 1992; 42(3):253–265Crossref, Medline, Google Scholar
68 Walker E, McGuire M, Bettes B: Recognition and identification of facial stimuli by schizophrenics and patients with affective disorders. Br. J. Clin Psychol 1984; 23(Pt 1):37–44Google Scholar
69 Feinberg TE, Rifkin A, Schaffer C, et al: Facial discrimination and emotional recognition in schizophrenia and affective disorders. Arch Gen Psych 1986; 43(3):276–279Crossref, Medline, Google Scholar
70 Kohler CG, Bilker W, Hagendoorn M, et al: Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol Psychiatry 2000; 48(2):127–136Crossref, Medline, Google Scholar
71 Borod JC, Martin CC, Alpert M, Brozgold A, Welkowitz J: Perception of facial emotion in schizophrenic and right brain-damaged patients. J Nerv Ment Dis 1993; 181(8):494–502Crossref, Medline, Google Scholar
72 Phillips ML, Williams L, Senior C, et al: A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res 1999; 92(1):11–31Crossref, Medline, Google Scholar
73 Schneider F, Gur RC, Gur RE, et al: Emotional processing in schizophrenia: neurobehavioral probes in relation to psychopathology. Schizophr Res 1995; 17(1):67–75Crossref, Medline, Google Scholar
74 Schneider F, Weiss U, Kessler C, et al: Differential amygdala activation in schizophrenia during sadness Schizophr Res 1998; 34(3):133–142Google Scholar
75 Phillips ML, Senior C, David AS: Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med 2000; 30(1):157–167Crossref, Medline, Google Scholar
76 Silbersweig DA, Stern E, Frith C, et al: A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378(6553):176–179Crossref, Medline, Google Scholar
77 David AS: Auditory hallucinations: phenomenology, neuropsychology and neuroimaging update. Acta Psychatr Scand Supple 1999; 395:95–104Crossref, Medline, Google Scholar
78 Stefanacci L, Amaral DG: Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. J Comp Neurol 2000; 421(1):52–79Crossref, Medline, Google Scholar
79 Carmichael ST, Price JL: Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1996; 363:615–641Crossref, Google Scholar
80 Saunders RC, Rosene DL, Van Hoesen GW: Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol 1988; 271:185–207Crossref, Medline, Google Scholar
81 Aggleton JP: A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res 1986; 64(3):515–526Crossref, Medline, Google Scholar
82 Bechara A, Damasio H, Tranel D, et al: Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci 1998; 18(1):428–437Crossref, Medline, Google Scholar
83 Baxter MG, Parker A, Lindner CC, et al: Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci 2000; 20(11):4311–4319Crossref, Medline, Google Scholar
84 Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP: Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus 1991; 1(2):207–220Crossref, Medline, Google Scholar
85 Nishijo H, Ono T, Nishino H: Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci 1988; 8:3570–3583Crossref, Medline, Google Scholar
86 Price JL: An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol 1973; 150(1):87–108Crossref, Medline, Google Scholar
87 Aggleton JP: A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res 1985; 57(2):390–399Crossref, Medline, Google Scholar
88 Price JL, Russchen FT, Amaral DG: The limbic region. II. The amygdaloid complex, in Handbook of Chemical Neuroanatomy. Edited by Hokfelt BT, Swanson LW. Amsterdam, Elsevier, 1987, pp 279–381Google Scholar
89 Bonda E: Organization of connections of the basal and accessory basal nuclei in the monkey amygdala [see comments]. Euro J Neurosci 2000; 12(6):1971–1992Crossref, Medline, Google Scholar
90 Amaral DG, Veazey RB, Cowan WM: Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Res 1982; 252(1):13–27Crossref, Medline, Google Scholar
91 Saper CB, Loewy AD: Efferent connections of the parabrachial nucleus in the rat. Brain Res 1980; 197(2):291–317Crossref, Medline, Google Scholar
92 Bernard JF, Alden M, Besson JM: The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 1993; 329:201–229Crossref, Medline, Google Scholar
93 Ricardo JA, Koh ET: Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978; 153(1):1–26Google Scholar
94 Rizvi TA, Ennis M, Behbehani MM, Shipley MT: Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J. Comp. Neurol. 1991; 303(1):121–131Google Scholar
95 Gray TS, Cassell MD, Williams TH: Synaptology of three peptidergic neuron types in the central nucleus of the rat amygdala. Peptides 1982; 3(3):273–281Crossref, Medline, Google Scholar
96 Wray S, Hoffman GE: Organization and interrelationship of neuropeptides in the central amygdaloid nucleus of the rat. Peptides 1983; 4(4):525–541Crossref, Medline, Google Scholar
97 Martin LJ, Powers RE, Dellovade TL, et al: The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol 1991; 309:445–485Crossref, Medline, Google Scholar
98 Veinante P, Freund-Mercier MJ: Distribution of oxytocin and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol 1997; 383:305–325Crossref, Medline, Google Scholar
99 Day HE, Curran EJ, Watson SJ, Jr., et al: Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1 beta. J Comp Neurol 1999; 413(1):113–128Crossref, Medline, Google Scholar
100 Merali Z, McIntosh J, Kent P, et al: Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 1998; 18(12):4758–4766Crossref, Medline, Google Scholar
101 Alheid GF, Heimer L: New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 1988; 27:1–39Crossref, Medline, Google Scholar
102 Swanson LW, Petrovich GD: What is the amygdala? [see comments]. Trends Neurosci 1998; 21(8):323–331Crossref, Medline, Google Scholar
103 Cassell MD, Freedman LJ, Shi C: The intrinsic organization of the central extended amygdala. Ann. N.Y. Acad. Sci. 1999; 877:217–241Google Scholar
104 deOlmos JS, Ingram WR: The projection field of the stria terminalis in the rat brain. J Comp Neurol 1972; 146:303–333Crossref, Medline, Google Scholar
105 Johnston JB: Further contributions to the study of the evolution of the forebrain. J Comp Neuro 1923; 35:337–481Crossref, Google Scholar
106 Heimer L, De Olmos JS, Alheid GF, et al: The human basal forebrain. Part II, in Handbook of Chemical Neuroanatomy, vol 15: The Primate Nervous System, Part III. Edited by Bloom FE, Bjorkland A, Hokfelt T. Amsterdam, Elsevier, 1999, pp 57–226Google Scholar
107 McDonald AJ: Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 1982; 208(4):401–418Crossref, Medline, Google Scholar
108 McDonald AJ: Neurons of the bed nucleus of the stria terminalis: a Golgi study in the rat. Brain Res Bull 1983; 10(1):111–120Crossref, Medline, Google Scholar
109 DeOlmos JS: Amygdala, in The Human Nervous System. Edited by Paxinos G. San Diego, Academic Press, 1990, pp 583–710Google Scholar
110 McDonald AJ: Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991; 44, (1):15–33Google Scholar
111 Krettek JE, Price JL: Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 1978; 178:225–254Crossref, Medline, Google Scholar
112 Weller KL, Smith DA: Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982; 232(2):255–270Google Scholar
113 Alden M, Besson J-M, Bernard J-F: Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: A PHA-L study in the rat. J. Comp Neurol 1994; 341:289–314Crossref, Medline, Google Scholar
114 Grove EA: Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol 1988; 277:315–346Crossref, Medline, Google Scholar
115 Fudge JL, Kunishio K, Walsh P, et al: Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience 2002; 110(2):257–275Crossref, Medline, Google Scholar
116 Fudge JL, Haber SN: The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience 2000; 97(3):479–494Crossref, Medline, Google Scholar
117 Fudge JL, Haber SN: Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience 2001; 104(3):807–827Crossref, Medline, Google Scholar
118 Heimer L: Basal forebrain in the context of schizophrenia. Brain Res. Brain Res Rev 2000; 31(2–3):205–235Google Scholar
119 Martina M, Royer S, Pare D: Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol 1999; 82(4):1843–1854Crossref, Medline, Google Scholar
120 Royer S, Martina M, Pare D: An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 1999; 19(23):10575–10583Crossref, Medline, Google Scholar
121 Collins DR, Pare D: Reciprocal changes in the firing probability of lateral and central medial amygdala neurons [published erratum appears in J Neurosci 1999 Apr 1;19(7):2841]. J Neurosci 1999; 19(2):836–844Crossref, Medline, Google Scholar
122 Kovelman JA, Sciebel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1621Medline, Google Scholar
123 Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR: Some cytoarchitectural abnormalities in the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 1991; 48:625–632Crossref, Medline, Google Scholar
124 Jakob H, Beckmann H: Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 1986; 65:303–326Crossref, Medline, Google Scholar
125 Falkai P, Bogerts B: Cell loss in hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol 1986; 236:154–161Google Scholar
126 Falkai P, Bogerts B, Schneider T, et al: Disturbed planum temporale asymmetry in schizophrenia. A quantitative post-mortem study. Schiz Res 1995; 14(2):161–176Crossref, Medline, Google Scholar
127 Benes FM, McSparran J, Bird ED, et al: Deficits in small interneurons in prefrontal and cingulate corticies of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48:990–1001Crossref, Google Scholar
128 Akbarian S, Bunney WE, Jr., Potkin SG, et al: Altered distribution of nicotamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe schizphrenics implies disturbances of cortical development. Arch Gen Psychiatry 1993; 50:169–177Crossref, Medline, Google Scholar
129 Selemon LD, Rajkowska G, Goldman-Rakic PS: Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol 1998; 392(3):402–412Crossref, Medline, Google Scholar
130 Barta PE, Pearlson GD, Brill LB, 2nd, et al: Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psych 1997; 154(5):661–667Crossref, Medline, Google Scholar
131 Flaum M, O'Leary DS, Swayze VW, 2nd, et al: Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res 1995; 29(4):261–276Crossref, Medline, Google Scholar
132 Arnold SE: Neurodevelopmental abnormalities in schizophrenia: insights from neuropathology. Dev Psychopath 1999; 11(3):439–456Crossref, Medline, Google Scholar
133 Rosenkranz JA, Grace AA: Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 1999; 19(24):11027–11039Crossref, Medline, Google Scholar
134 Rosenkranz JA, Grace AA: Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 2001; 21(11):4090–4103Crossref, Medline, Google Scholar
135 Rogan MT, Staubli UV, LeDoux JE: Fear conditioning induces associative long-term potentiation in the amygdala [see comments] [published erratum appears in Nature 1998 Feb 19;391(6669):818]. Nature 1997; 390(6660):604–607Crossref, Medline, Google Scholar
136 Maren S: Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci 1999; 22(12):561–567Crossref, Medline, Google Scholar
137 Huang YY, Kandel ER: Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron 1998; 21(1):169–178Crossref, Medline, Google Scholar
138 Li H, Weiss SR, Chuang DM, Post RM, Rogawski MA: Bidirectional synaptic plasticity in the rat basolateral amygdala: characterization of an activity-dependent switch sensitive to the presynaptic metabotropic glutamate receptor antagonist 2S-alpha-ethylglutamic acid. J Neurosci 1998. Mar. 1 1998; 18:1662–1670Google Scholar
139 Laruelle M, Abi-Dargham A: Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol 1999; 13(4):358–371Crossref, Medline, Google Scholar
140 Lieberman JA, Sheitman BB, Kinon BJ: Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 1997; 17(4):205–229Crossref, Medline, Google Scholar
141 Davis M, Hitchcock JM, Bowers MB, et al: Stress-induced activation of prefrontal cortex dopamine turnover: blockade by lesions of the amygdala. Brain Res 1994; 664(1–2):207–210Google Scholar
142 Louilot A, Besson C: Specificity of amygdalostriatal interactions in the involvement of mesencephalic dopaminergic neurons in affective perception. Neuroscience 2000; 96(1):73–82Crossref, Medline, Google Scholar
143 Maeda H, Mogenson GJ: Electrophysiological responses of neurons of the ventral tegmental area to electrical stimulation of amygdala and lateral septum. Neuroscience 1981; 6(3):367–376Crossref, Medline, Google Scholar
144 Rouillard C, Freeman AS: Effects of electrical stimulation of the central nucleus of the amygdala on the in vivo electrophysiological activity of rat nigral dopaminergic neurons. Synapse 1995; 21(4):348–356Crossref, Medline, Google Scholar



