In Vivo Proton Magnetic Resonance Spectroscopy of the Medial Temporal Lobes of Former Prisoners of War With and Without Posttraumatic Stress Disorder
Abstract
Proton magnetic resonance spectroscopy was used to compare medial temporal lobe (MTL) concentrations of N-acetylaspartate and choline between former prisoners of war (POWs) with and without posttraumatic stress disorder (PTSD). MTL N-acetylaspartate and reexperiencing symptoms correlated strongly in the POW subjects with PTSD, suggesting a relationship between reexperiencing symptoms and the integrity of MTL structures.
Among those exposed to traumatic experiences associated with combat, former prisoners of war (POWs) have been frequent subjects of research interest. Former POWs tend to have high incidence of posttraumatic stress disorder (PTSD). Studies suggest that more than 70% of former POWs meet lifetime criteria for PTSD.1 Far more than the 15%–18% of combat veterans typically meet criteria for chronic PTSD. A number of neuropsychological assessments of former POWs show evidence of impaired cognitive function related to severe weight loss;2 however, some investigators dispute this finding.3 Despite the psychiatric interest in this group, limited information is available regarding how the neurobiology of former POW subjects with PTSD might differ from their counterparts without PTSD. Given the overall level of interest in neuroimaging in PTSD, the lack of available information is somewhat surprising. PTSD subjects have been examined using magnetic resonance imaging (MRI),4 proton magnetic resonance spectroscopy,5 positron emission tomography (PET),6 and single photon emission computerized tomography (SPECT).7 Yet, to our knowledge, no investigator has used any of these techniques to examine former POW subjects. While this may be due to the limited availability of former POW populations, examining these groups offers certain advantages. One advantage is that former POW subjects provide insight into the effect of time passage on PTSD subjects versus the time passage effect on individuals who experienced similar traumas but do not meet criteria for PTSD. Substantive evidence indicates that many former POWs suffer from the effects of PTSD for many years;1 still, little is known about the long-term cerebral consequences of chronic PTSD.
Proton magnetic resonance spectroscopy (MRS) has been used to examine different PTSD populations, such as combat veterans8,9 and abused children.5 MRS can be used to detect in vivo amounts of various neurotransmitters and metabolites, including N-acetylaspartate (NAA), creatine (Cr), choline compounds (Cho), and other compounds in the brain within a defined volume or voxel. Reduced levels of NAA have been found in neurological diseases associated with neuronal damage and death,10 thus decreased levels of NAA have been considered symptomatic of decreased neuronal density and impaired neuronal health.11 Studies that examine NAA/Cr ratios in the medial temporal lobes (MTL) of combat veterans with PTSD have shown lower NAA/Cr ratios in the MTL of PTSD subjects, as compared with ratios among the controls.8,9
In this study, our objective involved comparing MRS determined levels of NAA and choline (Cho) in the MTL of former POW subjects who have PTSD with former POW subjects who do not have PTSD. This was done in order to determine whether the presence of PTSD would be associated with decreased MTL NAA ratios in subjects who were exposed to similar traumas and to compare MTL NAA ratios of former POWs who have histories of severe weight loss with those who do not, as significant differences in MRS-determined neurotransmitter ratios based on previous weight loss may complicate future findings. Given our previous findings,8 we predicted that we would discover lower NAA/Cr ratios in the MTL of POW subjects with PTSD.
METHODS
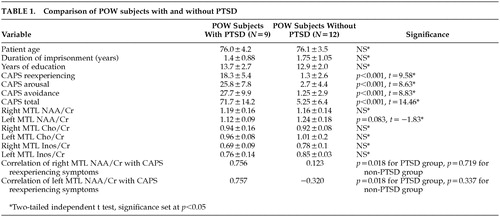
Twenty one male veteran POW subjects participated in the study. The Human Use Committee of the University of Arkansas for Medical Sciences approved the research protocol. Written informed consent was obtained from all participants. All subjects had combat experience and had been held captive during either World War II (WWII) or the Korean conflict (KC). The average length of imprisonment is noted in Table 1. Using stringent exclusion and inclusion criteria, research subjects were carefully selected. All participants were right-handed; and none had a history of traumatic brain injury with loss of consciousness, neurological impairment, degenerative neurological illness, or dementia. Moreover, none of the 21 study subjects met criteria for either current or lifetime alcohol dependence. The study was conducted on a voluntary basis, without compensation of any kind.
The presence of PTSD was ascertained with the use of the Clinician Administered PTSD Scale (CAPS-2).12 CAPS-2 scores were tallied for each subject in terms of the total score and symptom cluster scores (reexperiencing, avoidance, and arousal). The Structured Clinical Interview for DSM-IV13 was also administered in order to determine current and lifetime psychiatric diagnoses. Participants were recruited by letters sent to a large group of former POWs, living in the same region, who were part of a POW registry. Initally, they were interviewed by phone to determine whether they met criteria for inclusion and did not meet any of the exclusion criteria. Subjects were then interviewed by a psychiatrist, and their medical histories were reviewed. If they qualified to participate in the study, they were scheduled for MRS.
The MRS investigations were performed on a 1.5 Tesla General Electric Signa MRI system, with a standard head coil that used a slightly modified automated single-voxel proton brain spectroscopy exam (PROBE/SV) routine. Voxel locations in the right and left medial temporal lobes were chosen by an individual who was blind to the diagnostic status of the subjects. The voxel's anterior border was delineated by first identifying and localizing the anterior portion of the hippocampus using spin-echo MRI scans in the coronal plane. The most anterior part of the hippocampus (the head) was then centered in the forward plane of the voxel, and the voxel was extended 3 cm posteriorly. The head alignment was assessed using repeated transverse spin-echo MRI scans, and further corrections were not necessary following the initial head fixation. After localization of a 2×2×3 cm3 voxel using spin-echo MRI scans in three orthogonal planes, the magnetic field homogeneity and water suppression were optimized automatically. A set of water reference scans and a water-suppressed, stimulated echo spectroscopy (STEAM) spectrum were acquired using an echo time (TE) of 30 ms, a repetition time (TR) of 2 sec, a mixing time (TM) of 13.7 ms, 128 transients, and a spectral width of 2500 Hz in 2048 points. The data were transferred to an off-line SPARC (Sun Microsystems) workstation and processed automatically to correct the spectral phase, the effects of residual eddy currents, and thebaseline. An automated, Marquardt-fitting routine yielded ratios of the peak intensities of NAA and Cho to Cr. The spectra were rejected if the resolution between the Cho and Cr peaks was no less than 50% of the distance from the peak maximum to the apparent baseline or if obvious artifacts interfered. Additional details have been previously provided.8
RESULTS
In terms of left and right MTL NAA/Cr, Cho/Cr, or Inos/Cr ratios, initial comparisons between groups that were separated solely by a history of high weight loss (10 subjects lost >35% of body weight loss during confinement) and low weight loss (11 subjects lost <35% of body weight during confinement) revealed no differences
Significant correlations between left and right MTL NAA/Cr ratios and CAPS-2 reexperiencing symptoms were observed within the PTSD subjects but not within the group of POWs without PTSD. The correlation of left MTL NAA/Cr and CAPS-2 reexperiencing symptoms in PTSD subjects was 0.757 (Pearson r, p=0.018), and the correlation between right temporal lobe NAA/Cr and CAPS-2 reexperiencing symptoms in the same group was 0.756 (Pearson r, p=0.018). No correlations were found between CAPS-2 arousal or avoidance symptoms and medial temporal lobe NAA/Cr ratios within the PTSD group, nor were they found among any CAPS-2 symptoms within the non-PTSD group and NAA/Cr ratios (Table 1).
There was a trend towards variance between left medial temporal lobe NAA/Cr ratios between groups, with POWs who suffer from PTSD having the lower ratio (two-tailed independent t test, t=−1.83, p=0.083). No significant differences between groups, in terms of Inos/Cr or Cho/Cr ratios, were found. Differences between right and left medial temporal lobe NAA/Cr, Inos/Cr, or Cho/Cr were also unobserved.
DISCUSSION
Given the trend towards lower medial temporal NAA/CR ratios between former WW2 and KC POWs with and without PTSD, this study concurs with previous research that examines MRS-determined differences in MTL neurotransmitter ratios in subjects with PTSD.6 The MRS findings in this study are not as clear-cut as our previous findings in which we compared combat veterans with PTSD to a control group of substance abusers. This, however, may be secondary to the relatively small number of subjects and the similarly traumatic experiences of both subject groups in the present study. The strong correlation between bilateral MTL NAA/Cr ratios and reexperiencing symptoms in the POW group with PTSD was the most unforeseen outcome in this investigation, since to our knowledge this is the first such finding. Also germane, no significant differences in MRS ratios, based on weight loss during internment, were found among former POWs.
Reexperiencing symptoms, not avoidance or arousal symptoms, correlate with medial temporal lobe integrity as measured by MRS, which is consistent with the known function of medial temporal structures in memory processing/consolidation. This is especially the case regarding emotionally relevant memory.14 This finding supports current literature that suggests that medial temporal lobe structures are part of the neural substrate of PTSD symptoms, and MRS-measured indicators of MTL structural integrity may be associated with reexperiencing symptoms, specifically in subjects with PTSD. In order to better determine the relationship between medial temporal lobe structures and PTSD symptoms, additional studies that more closely compare POW subjects and PTSD subjects with MRS measures and MRI volumetric analyses of specific medial temporal lobe structures are underway. Studies that compare MRS measures, MRI-determined hippocampal volumes, and neuropsychological functioning of POWs (with and without PTSD) to nontraumatized, age-matched controls are also in progress.
 |
1 Sutker PB, Allain AN, Winstead DK: Psychopathology and psychiatric diagnoses of World War II Pacific theater prisoner of war survivors and combat veterans. Am J Psychiatry 1993; 150:240–245Crossref, Medline, Google Scholar
2 Sutker PB, Galina ZH, West JA, et al: Trauma induced weight loss and cognitive deficits among former prisoners of war. J Consult Clin Psychol 1990; 58:323–328Crossref, Medline, Google Scholar
3 Sulway MR, Broe GA, Creasey H, et al: Are malnutrition and stress risk factors for accelerated cognitive decline? a prisoner of war study. Neurology 1996; 46:650–655Crossref, Medline, Google Scholar
4 Bremner JD, Randall P, Scott TM, et al: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Crossref, Medline, Google Scholar
5 DeBellis MD, Keshavan MS, Spencer S, et al: N-acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry 2000; 157:1175–1177Crossref, Medline, Google Scholar
6 Bremner JD, Innis RB, Ng CK, et al: Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatr 1997; 54:246–254Crossref, Medline, Google Scholar
7 Zubieta JK, Chinitz JA, Lombardi U, et al: Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res 1999; 33:259–264Crossref, Medline, Google Scholar
8 Freeman TW, Cardwell D, Karson CN, et al: In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Mag Reson Med 1998; 40:66–71Crossref, Medline, Google Scholar
9 Schuff M, Marmar CR, Weiss DS, et al: Reduced hippocampal volume and N-acetylaspartate in posttraumatic stress disorder. Ann NY Acad Sci 1997; 821:516–520Crossref, Medline, Google Scholar
10 Brooks WM, Sabet A, Sibbitt WL, et al: Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol 1997; 24:2323–2329Medline, Google Scholar
11 Brooks WM, Jung RE, Ford CC, et al: Relationship between neurometabolite derangement and neurocognitive dysfunction in systemic lupus erythematosus. J Rheumatol 1999; 26:81–85Medline, Google Scholar
12 Weathers F, Keane T, Davidson JR: Clinician-administered PTSD scale: a review of the first 10 years of research. Depress Anxiety 2001; 13:132–156Crossref, Medline, Google Scholar
13 First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
14 Bremner JD: Does stress damage the brain? Biol Psychol 1999; 45:797–805Crossref, Google Scholar



