An Open-Label Trial of Donepezil (Aricept) in the Treatment of Persons With Mild Traumatic Brain Injury
SIR: We read with interest, the review by Griffin et. al.1 on the use of cholinergic agents in the treatment of persons who sustained traumatic brain injury (TBI). As early as 1997 (Poster session at the joint meeting of the American Neuropsychiatric Association and the British Neuropsychiatry Association. Cambridge, England.), we too suspected that these medications might be beneficial in treating cognitive dysfunction, memory deficits, and emotional instability, all observed in TBI.
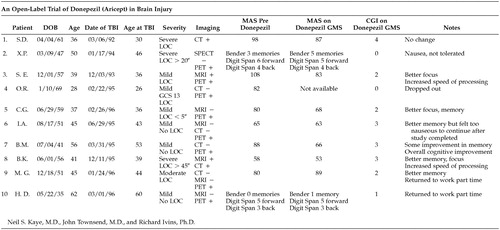
Our findings revealed the following Table 1:
An Open-Label Trial of Donepezil (Aricept) in the Treatment of Persons with Mild Traumatic Brain Injury
Methods:
In an open label trial, subjects who participated in this pilot program were the first 10 consecutive patients known to have sustained TBI and received treatment in one outpatient practice after the release of donepezil. Patients were given 5 mg daily for the first 4 weeks and 10 mg daily for the second 4 weeks. Pretreatment and posttreatment neuropsychiatric evaluations, including clinical global improvement (CGI) ratings and a symptom focused neuropsychological test battery, were used to measure responses to the medication.
Results:
Eighty percent (8/10) of the subjects completed the study. One subject withdrew due to noncompliance, and another dropped out out due to intolerable GI side effects of nausea. This heterogeneous group ranged in age from 26–60 years, with a mean of 41 years. Severity of head injury included mild (6 cases), moderate (1 case), and severe (3 cases). Time since head injury ranged from 1 to 5 years, with a mean of 1.2 years.
Discussion:
Although we predicted improvement in the domain of memory, we were unable to document any such positive change. The Global Memory Scale (GMS) of the Memory Assessment Scale (MAS) does not seem to improve (MAS margin of error is +/−4 points.) However, CGI's conducted by two independent raters showed improvement. Patients also rated themselves somewhat improved in most cases, but not necessarily in the memory domain as expected. The overall impression was that they had improved focus, attention and clarity of thought while on the medication. A number of patients commented that their speed of processing appeared to be better or they were able to keep multiple ideas in mind simultaneously. Family members frequently described improved socialization.
We are encouraged by the results and believe that further study of donepezil in TBI is indicated. We would recommend assessing change by using other test instruments, such as the Categories Test, which is better suited to assess cognitive flexibility, nonverbal abstract reasoning, learning from mistakes, and incidental visual memory. In addition, a larger trial should, as a minimum, control for age, severity of injury, time since injury, and intelligence quotient (IQ). A subsequent controlled trial that uses traditional blinding methods and placebo could follow if warranted.
Of note, is that at the end of the study, seven of the eight subjects who completed the study (88%) elected to remain on donepezil, as they believed it was efficacious. The only subject who completed the study and did not elect to stay on the medication reported a positive effect on memory, but experienced nausea as a side effect.
Conclusion:
Testing Donepezil—which is approved, available, and well tolerated—in a patient population that historically has limited progress should prove useful. As in Alzheimer's disease (AD), the acetylcholinesterase inhibitors may be of greater benefit in the overall functioning of the individual than the specific domain of memory.2
Dr. Base-XML-tag-lib speaks for Pfizer Phamaceuticals. He has never owned stock in the corporation and has never been an employee. No funding from any outside source was used in this study.
 |
1 Griffin S, et al: A review of cholinergic agents in the treatment of neurobehavioral deficits following traumatic brain injury. J Neuropsychiatry Clin Neurosci 2003; 15:1, 17–26Crossref, Medline, Google Scholar
2 Rogers S, et al: Donepezil improves cognition and global function in Alzheimer disease. Arch Intern Med 1998; 14:197–230Google Scholar



