Apathy is Associated With Volume of the Nucleus Accumbens in Patients Infected With HIV
Abstract
Apathy refers to a reduction in self-initiated behavior, and it is commonly reported by patients infected with human immunodeficiency virus (HIV). It remains unclear whether apathy among HIV patients reflects a direct effect of the virus on subcortical brain circuits or a secondary neuropsychiatric symptom. In the present study we examined the relationship between ratings of apathy and quantitative analysis of the nucleus accumbens (NA), a subcortical brain structure that regulates initiation of behavioral activation. Twelve HIV-positive individuals without dementia were administered the Marin Apathy Scale and underwent neuroimaging. Voxel-based quantification of the nucleus accumbens was completed using a segmentation protocol. Results of our study revealed that increased ratings of apathy were significantly correlated with lower volume of the nucleus accumbens. By contrast, ratings of depression were unrelated to either apathy or nucleus accumbens volume. These findings provide preliminary evidence that apathy reflects direct involvement of the central nervous system in patients with HIV.
Neuropsychiatric sequelae of human immunodeficiency virus (HIV) have been a primary focus of clinical research since the beginning of the epidemic. Depression has received the most concentrated scientific focus, due in part to the high prevalence of depression in HIV and the clinical importance of this condition. However, individuals infected with HIV exhibit additional neuropsychiatric symptoms including mania, anxiety, and psychotic disorders.1 Apathy is another neuropsychiatric symptom that is reported more commonly among HIV patients than the general population.1–4
Apathy refers to a reduction in self-initiated cognitive, emotional, and behavioral activity. Symptoms of apathy overlap with features of depression, but the two conditions are unique and can be reliably differentiated in patient samples.5 Apathy has long been recognized as a potential feature of AIDS dementia complex, along with other symptoms of subcortical dysfunction including memory impairment, poor concentration, and reduced psychomotor speed.6 More recent studies have reported elevated rates of apathy in symptomatic patients without dementia,2 and among patients with less severe disease progression. For example, we recently reported7 that approximately 26% of HIV patients exhibit clinically significant apathy. Importantly, the average CD4 cell count of this sample was greater than 300, suggesting that apathy is present even among patients without severe immune suppression.
The etiology of apathy associated with HIV has not been determined. While it is possible that apathy develops secondary to the psychosocial demands associated with the disease, it is also possible that apathy develops secondary to direct effects of the virus on cerebral function. HIV enters the nervous system soon after initial infection and subsequently aggregates in the basal ganglia.8–10 The basal ganglia and related structures are critical nuclei in parallel frontal subcortical circuits that in part regulate cognition, emotions, and behavioral activation.11–12 Previous studies of apathy in HIV have demonstrated significant correlations between cognitive impairment and increased ratings of apathy, suggesting that the virus may negatively impact shared subcortical systems.2–3 While these findings are intriguing, not all studies have reported significant relationships between cognitive impairment and apathy in HIV4 and evidence that apathy results from direct neural abnormalities has been lacking.
In the present study we examined the relationship between the volume of the nucleus accumbens and ratings of apathy in HIV. The nucleus accumbens was selected for the volumetric study because this nucleus is anatomically located in the region of greatest HIV concentration,13 and the nucleus is a central structure in the anterior cingulate frontal subcortical circuit. This circuit is involved in the initiation of behavior and emotional regulation, and lesions to this circuit produce prominent symptoms of apathy.11–12 In the present study we predicted that ratings of apathy would be significantly correlated with reduced volume of the nucleus accumbens.
METHODS
Subjects
A total of 12 (6 men and 6 women) HIV-positive individuals enrolled in the study. All participants were recruited from an academic HIV care program. HIV diagnosis was based on serologic testing by ELISA and Western blot. Patients with a history of bipolar disorder or schizophrenia, neurologic disorder, learning disability, or developmental disability were excluded from the study. Patients were not excluded if they reported a history of substance abuse or head injury. All of the HIV patients were infected with the virus through intraveneous drug use. Three of the twelve subjects were currently using alcohol and illicit drugs including heroin and/or cocaine. No subjects were in acute withdrawal. No subjects reported a history of significant head injury. The patients averaged 42.7 (5.7) years of age, 12.5 (2.7) years of education, 95.5 (63.7) months since diagnosis. The median CD4 cell count was 249 (range = 24-312). All patients were taking ART and all were asymptomatic. No patients had been diagnosed with AIDS.
Twenty-five healthy comparison subjects were included to contrast apathy scores. Healthy comparison subjects reported no significant history of head injury (defined as loss of consciousness > 15 min.), substance abuse, or psychiatric/neurologic illness. The healthy comparison subjects averaged 38.6 (11.2) years of age and 15.5 (2.7) years of education.
Procedure
Participants completed demographic questionnaires and self-report measures of apathy and mood in a single testing session and neuroimaging was conducted within one week of the initial evaluation. All measures were administered and scored according to standard procedures. Participants received financial compensation for participation in the study. Written informed consent was obtained prior to enrollment. The protocol was approved by the local IRB.
Apathy.
Apathy was measured by the Apathy Evaluation Scale- Self-report version (AES; 14). This measure is an 18-item self-report scale of apathy behavior that with good psychometric properties. Individuals are required to respond to their degree of agreement with each question using a four point Likert scale. Higher scores reflect greater apathy (1 = not at all and 4 = extremely). Total score was the dependent measure.
Mood.
The Chicago Multicomponent Depression Inventory was administered to assess mood (CMDI: 15). The CMDI was developed to avoid the confounding effects of physical symptoms (e.g., feeling tired, difficulty sleeping) which are common in diseases that involve the immune system. The CMDI was administered in the present study as previous investigations have reported that scores from the Beck Depression Inventory and related global measures of depression confound somatic from nonsomatic symptoms of depression in HIV.3 The measures consists of a 42-item self-report inventory that separately assesses mood (e.g., “I’m sad”), self-evaluative (“I’m worthless”), and somatic (“I’m tired”) symptoms of depression. Each subscale consists of 14 items and subjects are required to respond using a 5-point Likert scale where 1 = “not at all” and 5= “extremely.” The individual subscales have strong reliability and validity, and each correlates significantly with clinical measures of depression.15 Total score on the mood subscale was the dependent variable for the present study. Higher scores reflect significant endorsement of depressive symptoms. A total score greater than 24 on the mood scale reflects clinically significant depressive symptoms.15
Magnetic Resonance Imaging
Neuroimaging was conducted on the HIV-positive sample; healthy comparison subjects did not undergo imaging. Neuroimaging was conducted using a 1.5 Tesla GE scanner with 5mm thin sections oriented parallel to the canthomeatal line. Interscan gap was 2 mm. Anatomical segmentation of the accumbens area was conducted using coronal T2-weighted TIRM data (25 slices, voxel dimensions = 1mm x 1mm x 6.05mm) and the computer program, CardViews.16 Prior to segmentation, data underwent positional normalization relative to the anterior and posterior commissures, as well as the interhemispheric fissure as defined at the level of the posterior commissure in the coronal plane.17 Intensity histograms were generated, within CardViews, to define borders for the lateral ventricles and caudate nuclei, as described in further detail by Filipek et al.17 The accumbens area was defined using both an intensity contour algorithm and previously established conventions.18 Intensity histograms were generated within CardViews.
Statistical Analyses.
Correlational analyses were conducted for the HIV positive group to examine the associations between apathy, mood disturbance and volume of the nucleus accumbens and the caudate nucleus. Since apathy was not normally distributed among either patient groups, nonparametric statistics were conducted for all analyses in which apathy was included. Finally, a regression was conducted to determine whether nucleus accumbens volume predicted apathy scores after controlling for duration of illness.
RESULTS
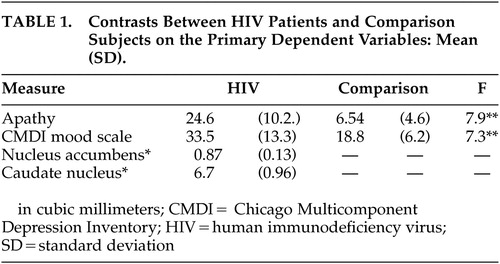
Ratings of apathy were significantly elevated among HIV patients compared to the healthy comparison subjects (Mann-Whitney U = 13.0, p < 0.05). Similarly, ratings of depressed mood were significantly higher among the HIV patients compared to healthy comparison subjects (t (36) = -3.5, p < 0.05). A total of 72% of the HIV patients reported scores on the depression measure that exceeded the clinical cutoff for clinically meaningful depression. Apathy and mood ratings were not significantly correlated among the HIV participants (r = 0.15, p > 0.05). Correlational analyses between the neuroimaging variables revealed a strong but nonsignificant relationship between caudate volume and nucleus accumbens volume (r = 0.48, p > 0.05). Note that this medium effect size would likely have been significant with a slightly larger sample size.
Correlation analyses between neuroimaging variables and ratings of apathy revealed that ratings of apathy were significantly related to volume of the nucleus accumbens (r = -0.59, p < 0.05). A strong relationship was also evident between ratings of apathy and the volume of the caudate nucleus (r = - 0.32), but the correlation was not statistically significant, largely due to the small sample size. Scores on the CMDI mood scale were not significantly related to volume of either structure (rs > 0.25, p < 0.05). Peripheral CD4 cell count was unrelated to the volume of either brain structure or severity of apathy. The regression analysis also revealed that nucleus accumbens volume was significantly associated with ratings of apathy after accounting for disease duration (p < 0.05).
DISCUSSION
The results of our study provide preliminary evidence that apathy is related to neural changes induced by HIV. Apathy was elevated among the sample of HIV patients compared to healthy comparison subjects, and the severity of apathy strongly correlated with the volume of the nucleus accumbens. The finding is highly consistent with current neuroanatomical models of brain circuitry and function.
Alexander and colleagues described the presence of five parallel circuits connecting the frontal lobes to subcortical brain structures.19–21 These circuits connect subcortical nuclei with cortical regions. Three of these circuits (dorsolateral prefrontal, lateral orbital frontal, and anterior cingulate) are involved in the regulation of cognitive and neuropsychiatric behavior. In each circuit, neural processing projects from the cortex through the striatum, then to the globus pallidus and, eventually to specific thalamic nuclei. Reciprocal connections project back to the cortex, resulting in “closed” functional loops. The caudate nucleus is the critical subcortical nucleus in the dorsolateral and orbital frontal circuits, while the nucleus accumbens is the primary subcortical structure in the anterior cingulate circuit. While this characterization of the subcortical circuitry is likely oversimplified, the linear relationships described within and between each system provides a useful model to test the effects of disease on neurobehavioral and neuropsychiatric function.
An apathetic behavioral syndrome has previously been associated with disruption to the frontal cortex;22–23 however, there is also evidence that similar behavioral alterations occur with disruption to the frontal-subcortical anterior cingulate circuitry. Specifically, behavioral changes such as apathy, withdrawal, and loss of initiative arise from damage not only to the frontal lobes but also to the ventral striatum or other areas of the loop. This striatal syndrome characterized by apathy has been associated with pathologically confirmed bilateral damage to the nucleus accumbens and adjacent structures (e.g., rostroventral globus pallidus, septal gray matter;24–25 Thus, the classic apathetic behavioral syndrome seen in frontal lobe anterior cingulate lesions also occurs with damage to the nucleus accumbens. This model supports the contention that apathy in HIV is associated with disruption of subcortical function secondary to the effects of the virus.
Previous efforts2–4 to determine whether apathy results from subcortical dysfunction in HIV examined the relationship apathy and severity of cognitive impairment, since the latter was also believed to result from disruption of frontal subcortical circuits (the dorsolateral circuit in particular). Two of these studies reported modest relationships between apathy and cognitive dysfunction, a finding not surprising given the neuroanatomical proximity of the caudate and the nucleus accumbens. More robust relationships between the two symptoms were likely not observed because apathy and cognitive dysfunction may emanate from different subcortical circuits. This difference could account for the lack of significant relationship between apathy and caudate volume in the present study, and it explains the significant relationship between volume of the caudate nucleus and severity of dementia in HIV patients reported in previous studies.26–27
The cross-sectional nature of our data do not definitely conclude that HIV directly impacts the function and structure of the nucleus accumbens, and in turn produces increased apathy. A longitudinal study that includes a more robust acquisition sequence for the MRI analyses and imaging of healthy comparison subjects is needed to determine whether the nucleus accumbens is atrophic in patients with HIV and whether these relationships exist among patients without a significant history of substance abuse. Until these studies are completed, the specific underlying process associated with the development of apathy in HIV will remain conjecture. Nevertheless, the fact that apathy scores were not elevated among the healthy comparison group in the present study provides some assurance that the relationship between apathy and morphometry of the nucleus accumbens is not a general relationship that exists independent of HIV.
Additional work is needed to more comprehensively examine the clinical and functional relevance of brain volume and neuropsychiatric symptoms in HIV. In particular, is important to examine morphometric changes to the nucleus accumbens relative to demographically similar healthy comparison group, and whether measures of central viral load correlate with the volume measurements. It is also important to determine the course of change that occurs in volumetrics as a function of illness duration, age, treatment exposure and treatment adherence. These factors are almost certainly critical in determining the overall impact of HIV on brain function. A more comprehensive investigation of these factors will lead to a better understanding of the impact of HIV on the expression of neuropsychiatric symptoms.
ACKNOWLEDGMENTS
This study was supported by grants awarded to Drs. Robert Paul (K23MH65857-01; RO3DA15045-01) and Bradford Navia (NS36524).
This study was also supported in part by NIMH K23MH65857 to Dr. Paul and NS36524 to Dr. Navia.
The authors acknowledge the support of the T32 Training Grant from the National Institute on Drug Abuse to the Miriam Hospital (T32DA13911) and the Miriam Center for AIDS Research.
 |
1 Hinkin CH, Castellon SA, Atkinson JH, et al: Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol 2001; 54: S44-S52Google Scholar
2 Castellon SA, Hinkin CH, Myers HF: Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc 2000; 6: 336–347Google Scholar
3 Castellon SA, Hinkin CH, Wood S, et al: Apathy, depression, and cognitive performance in HIV-1 infection. J Neuropsychiatry Clin Neurosci. 1998; 10320–10329Google Scholar
4 Rabkin JG, Ferrando SJ, van Gorp W, et al: Relationships among apathy, depression and cognitive impairment in HIV/AIDS. J Neuropsych Clin Neurosci 2000; 12: 451–457Google Scholar
5 Marin RS: Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147: 22–30Google Scholar
6 Ho DD, Bredesen DE, Vinters HV, et al: The acquired immunodeficiency syndrome (AIDS) dementia complex. Ann Intern Med. 1989;111:400–410Google Scholar
7 Tate D, Paul R, Flanigan T, et al: Impact of apathy on quality of life in HIV. AIDS Patient Care (in press)Google Scholar
8 Nath A: Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 2002;186 2:S193–198Google Scholar
9 Clements JE, Babas T, Mankowski JL, et al: The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis 2002;186:905–913Google Scholar
10 Neuen-Jacob E, Arendt G, Wendtland B, et al: Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin Neuropathol 1993;12(6):315–24.Google Scholar
11 Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50: 873–880Google Scholar
12 Tekin S, Cummings JL: Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002; 53:647–654.Crossref, Medline, Google Scholar
13 Wiley CA, Soontornniyomkij V, Radhakrishnan L, et al: Distribution of brain HIV load in AIDS. Brain Pathol 1998;8:277–284Google Scholar
14 Marin RS, Biedrzycki RC, Firincigullari S: Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research 1991; 38: 143–162Google Scholar
15 Nyehnuis DL, Luchetta T, Yamamoto C, et al: The development, standardization, and initial validation of the Chicago Multiscale Depression Inventory. J Person Assess 1998; 70: 386–401Google Scholar
16 Caviness VS Jr, Kennedy DN, Richelme C, et al: The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex 1996;6 (5):726–736Google Scholar
17 Filipek PA, Richelme C, Kennedy DN, et al: The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex 1994 Jul-Aug;4(4):344–360Google Scholar
18 Makris N, Meyer JW, Bates JF, et al: MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 1999; 9:18–45Crossref, Medline, Google Scholar
19 Alexander GE, Crutcher MD, DeLong MR: Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1990;85:119–146Google Scholar
20 Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381Google Scholar
21 Alexander GE, Crutcher MD: Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Alexander Trends Neurosci 1990 Jul;13(7):266–271Google Scholar
22 Okada K, Kobayashi S, Yamagata S, et al: Poststroke apathy and regional cerebral blood flow. Stroke 1997; 28: 2437–2441Google Scholar
23 Andersson S, Bergedalen AM: Cognitive correlates of apathy in traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:184–191Google Scholar
24 Cohen RA, Kaplan RF, Zuffante P, et al: Alteration of intention and self-initiated action associated with bilateral anterior cingulotomy. J Neuropsychiatry Clin Neurosci 1999;11:444–453Google Scholar
25 Mega MS, Cummings JL: Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994;6(4):358–370Google Scholar
26 Paul R, Cohen R, Navia B, et al: Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 2002 May; 26:353–359Google Scholar
27 Aylward EH, Henderer JD, McArthur JC, et al: Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 1993; 43:2099–104Crossref, Medline, Google Scholar



